Groups containing oxygen edit
Compounds that contain C-O bonds each possess differing reactivity based upon the location and hybridization of the C-O bond, owing to the electron-withdrawing effect of sp² hybridized oxygen and the donating effects of sp³ hybridized oxygen.
| Chemical class | Group | Formula | Structural Formula | Prefix | Suffix | Example |
|---|---|---|---|---|---|---|
| Ether | Ether | ROR' | 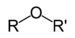 | alkoxy- | alkyl alkyl ether |  Diethyl ether (Ethoxyethane) |
| Alcohol | Hydroxyl | ROH |  | hydroxy- | -ol | 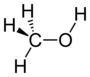 Methanol |
| Peroxide | Peroxy | ROOR |  | peroxy- | alkyl peroxide |  Di-tert-butyl peroxide |
| Hydroperoxide | Hydroperoxy | ROOH |  | hydroperoxy- | alkyl hydroperoxide | 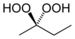 Methyl ethyl ketone peroxide |
| Ketone | Carbonyl | RCOR' | 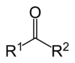 | keto-, oxo- | -one | 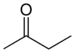 Methyl ethyl ketone (Butanone) |
| Aldehyde | Aldehyde | RCHO |  | aldo- | -al |  Acetaldehyde (Ethanal) |
| Acyl halide | Haloformyl | RCOX | 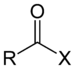 | haloformyl- | -oyl halide | 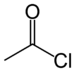 Acetyl chloride (Ethanoyl chloride) |
| Ester | Ester | RCOOR' | 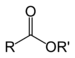 | alkyl alkanoate |  Ethyl butyrate (Ethyl butanoate) | |
| Carboxylic acid | Carboxyl | RCOOH | 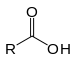 | carboxy- | -ic acid | 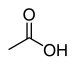 Acetic acid (Ethanoic acid) |
| Carboxylate | Carboxylate | RCOO− | 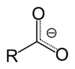 | carboxy- | -oate |  Sodium acetate (Sodium ethanoate) |
| Carbonate | Carbonate ester | ROCOOR | 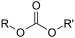 | alkyl carbonate |
🔥 Top keywords: Akademia e Shkencave e RPS te ShqiperiseAlexandria Ocasio-CortezBilderberg GroupCristiano RonaldoDong XiaowanMinecraftOperation GladioPrimal cutRiot FestStrictly Come Dancing (series 7)Main PageSpecial:SearchWikipedia:Featured picturesIga ŚwiątekRamoji RaoHit Man (2023 film)Pawan KalyanMichael Mosley (broadcaster)2024 Indian general electionWilliam AndersChirag PaswanCleopatraJasmine PaoliniPat SajakProject 20252024 ICC Men's T20 World CupDeaths in 2024Glen PowellKidnapping of Noa ArgamaniAdria ArjonaBad Boys: Ride or DieEarthriseVanna WhiteThe Acolyte (TV series)UEFA Euro 2024YouTubeThe Watchers (film)Beechcraft T-34 MentorSabrina CarpenterNormandy landingsBarry Keoghan.xxxGodzilla Minus OneTaylor SwiftN. Chandrababu NaiduClash at the Castle: ScotlandICC Men's T20 World CupTom Jones (singer)LAC Colombia Flight 028
