Thallium (81Tl) has 41 isotopes with atomic masses that range from 176 to 216. 203Tl and 205Tl are the only stable isotopes and 204Tl is the most stable radioisotope with a half-life of 3.78 years. 207Tl, with a half-life of 4.77 minutes, has the longest half-life of naturally occurring Tl radioisotopes. All isotopes of thallium are either radioactive or observationally stable, meaning that they are predicted to be radioactive but no actual decay has been observed.
| |||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Tl) | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Thallium-202 (half-life 12.23 days) can be made in a cyclotron[4] while thallium-204 (half-life 3.78 years) is made by the neutron activation of stable thallium in a nuclear reactor.[5]
In the fully ionized state, the isotope 205Tl becomes beta-radioactive, decaying to 205Pb,[6] but 203Tl remains stable.
205Tl is the decay product of bismuth-209, an isotope that was once thought to be stable but is now known to undergo alpha decay with an extremely long half-life of 2.01×1019 y.[7] 205Tl is at the end of the neptunium series decay chain.
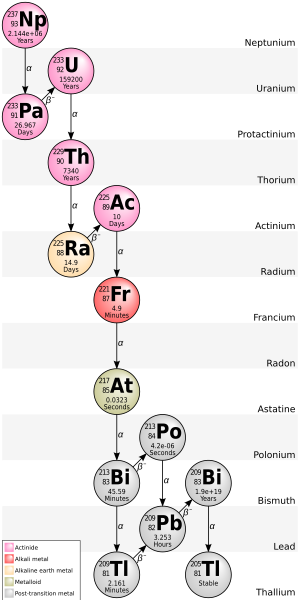
List of isotopes
edit| Nuclide[8] [n 1] | Historic name | Z | N | Isotopic mass (Da)[9] [n 2][n 3] | Half-life [n 4] | Decay mode [n 5] | Daughter isotope [n 6] | Spin and parity [n 7][n 4] | Natural abundance (mole fraction) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Excitation energy[n 4] | Normal proportion | Range of variation | |||||||||||||||||
| 176Tl[10] | 81 | 95 | 176.00059(21)# | 2.4+1.6 −0.7 ms | p (~63%) | 175Hg | (3−, 4−, 5−) | ||||||||||||
| α (~37%) | 172Au | ||||||||||||||||||
| 176mTl | ~671 keV | 290+200 −80 μs | p (~50%) | 175Hg | |||||||||||||||
| α (~50%) | 172mAu | ||||||||||||||||||
| 177Tl[11] | 81 | 96 | 176.996427(27) | 18(5) ms | α (73%) | 173Au | (1/2+) | ||||||||||||
| p (27%) | 176Hg | ||||||||||||||||||
| 177mTl | 807(18) keV | 230(40) μs | p (51%) | 176Hg | (11/2−) | ||||||||||||||
| α (49%) | 173Au | ||||||||||||||||||
| 178Tl[12] | 81 | 97 | 177.99490(12)# | 255(9) ms | α (62%) | 174Au | (4-,5-) | ||||||||||||
| β+ (38%) | 178Hg | ||||||||||||||||||
| β+, SF (0.15%) | (various) | ||||||||||||||||||
| 179Tl[13] | 81 | 98 | 178.99109(5) | 437(9) ms | α (60%) | 175Au | (1/2+) | ||||||||||||
| β+ (40%) | 179Hg | ||||||||||||||||||
| 179m1Tl | 825(10)# keV | 1.41(2) ms | α | 175Au | (11/2−) | ||||||||||||||
| IT (rare) | 179Tl | ||||||||||||||||||
| β+ (rare) | 179Hg | ||||||||||||||||||
| 179m2Tl | 904.5(9) keV | 119(14) ns | IT | 179Tl | (9/2−) | ||||||||||||||
| 180Tl[14] | 81 | 99 | 179.98991(13)# | 1.09(1) s | β+ (93%) | 180Hg | 4-# | ||||||||||||
| α (7%) | 176Au | ||||||||||||||||||
| β+, SF (0.0032%) | 100Ru, 80Kr[15] | ||||||||||||||||||
| 181Tl[16] | 81 | 100 | 180.986257(10) | 2.9(1) s | β+ (91.4%) | 181Hg | 1/2+# | ||||||||||||
| α (8.6%) | 177Au | ||||||||||||||||||
| 181mTl | 834.9(4) keV | 1.40(3) ms | IT (99.60%) | 181Tl | (9/2−) | ||||||||||||||
| α (0.40%) | 177Au | ||||||||||||||||||
| 182Tl | 81 | 101 | 181.98567(8) | 2.0(3) s | β+ (96%) | 182Hg | 2−# | ||||||||||||
| α (4%) | 178Au | ||||||||||||||||||
| 182m1Tl | 100(100)# keV | 2.9(5) s | α | 178Au | (7+) | ||||||||||||||
| β+ (rare) | 182Hg | ||||||||||||||||||
| 182m2Tl | 600(140)# keV | 10− | |||||||||||||||||
| 183Tl | 81 | 102 | 182.982193(10) | 6.9(7) s | β+ (98%) | 183Hg | 1/2+# | ||||||||||||
| α (2%) | 179Au | ||||||||||||||||||
| 183m1Tl | 630(17) keV | 53.3(3) ms | IT (99.99%) | 183Tl | 9/2−# | ||||||||||||||
| α (.01%) | 179Au | ||||||||||||||||||
| 183m2Tl | 976.8(3) keV | 1.48(10) μs | (13/2+) | ||||||||||||||||
| 184Tl | 81 | 103 | 183.98187(5) | 9.7(6) s | β+ | 184Hg | 2−# | ||||||||||||
| 184m1Tl | 100(100)# keV | 10# s | β+ (97.9%) | 184Hg | 7+# | ||||||||||||||
| α (2.1%) | 180Au | ||||||||||||||||||
| 184m2Tl | 500(140)# keV | 47.1 ms | IT (99.911%) | (10−) | |||||||||||||||
| α (.089%) | 180Au | ||||||||||||||||||
| 185Tl | 81 | 104 | 184.97879(6) | 19.5(5) s | α | 181Au | 1/2+# | ||||||||||||
| β+ | 185Hg | ||||||||||||||||||
| 185mTl | 452.8(20) keV | 1.93(8) s | IT (99.99%) | 185Tl | 9/2−# | ||||||||||||||
| α (.01%) | 181Au | ||||||||||||||||||
| β+ | 185Hg | ||||||||||||||||||
| 186Tl | 81 | 105 | 185.97833(20) | 40# s | β+ | 186Hg | (2−) | ||||||||||||
| α (.006%) | 182Au | ||||||||||||||||||
| 186m1Tl | 320(180) keV | 27.5(10) s | β+ | 186Hg | (7+) | ||||||||||||||
| 186m2Tl | 690(180) keV | 2.9(2) s | (10−) | ||||||||||||||||
| 187Tl | 81 | 106 | 186.975906(9) | ~51 s | β+ | 187Hg | (1/2+) | ||||||||||||
| α (rare) | 183Au | ||||||||||||||||||
| 187mTl | 335(3) keV | 15.60(12) s | α | 183Au | (9/2−) | ||||||||||||||
| IT | 187Tl | ||||||||||||||||||
| β+ | 187Hg | ||||||||||||||||||
| 188Tl | 81 | 107 | 187.97601(4) | 71(2) s | β+ | 188Hg | (2−) | ||||||||||||
| 188m1Tl | 40(30) keV | 71(1) s | β+ | 188Hg | (7+) | ||||||||||||||
| 188m2Tl | 310(30) keV | 41(4) ms | (9−) | ||||||||||||||||
| 189Tl | 81 | 108 | 188.973588(12) | 2.3(2) min | β+ | 189Hg | (1/2+) | ||||||||||||
| 189mTl | 257.6(13) keV | 1.4(1) min | β+ (96%) | 189Hg | (9/2−) | ||||||||||||||
| IT (4%) | 189Tl | ||||||||||||||||||
| 190Tl | 81 | 109 | 189.97388(5) | 2.6(3) min | β+ | 190Hg | 2(−) | ||||||||||||
| 190m1Tl | 130(90)# keV | 3.7(3) min | β+ | 190Hg | 7(+#) | ||||||||||||||
| 190m2Tl | 290(70)# keV | 750(40) μs | (8−) | ||||||||||||||||
| 190m3Tl | 410(70)# keV | >1 μs | 9− | ||||||||||||||||
| 191Tl | 81 | 110 | 190.971786(8) | 20# min | β+ | 191Hg | (1/2+) | ||||||||||||
| 191mTl | 297(7) keV | 5.22(16) min | β+ | 191Hg | 9/2(−) | ||||||||||||||
| 192Tl | 81 | 111 | 191.97223(3) | 9.6(4) min | β+ | 192Hg | (2−) | ||||||||||||
| 192m1Tl | 160(50) keV | 10.8(2) min | β+ | 192Hg | (7+) | ||||||||||||||
| 192m2Tl | 407(54) keV | 296(5) ns | (8−) | ||||||||||||||||
| 193Tl | 81 | 112 | 192.97067(12) | 21.6(8) min | β+ | 193Hg | 1/2(+#) | ||||||||||||
| 193mTl | 369(4) keV | 2.11(15) min | IT (75%) | 193Tl | 9/2− | ||||||||||||||
| β+ (25%) | 193Hg | ||||||||||||||||||
| 194Tl | 81 | 113 | 193.97120(15) | 33.0(5) min | β+ | 194Hg | 2− | ||||||||||||
| α (10−7%) | 190Au | ||||||||||||||||||
| 194mTl | 300(200)# keV | 32.8(2) min | β+ | 194Hg | (7+) | ||||||||||||||
| 195Tl | 81 | 114 | 194.969774(15) | 1.16(5) h | β+ | 195Hg | 1/2+ | ||||||||||||
| 195mTl | 482.63(17) keV | 3.6(4) s | IT | 195Tl | 9/2− | ||||||||||||||
| 196Tl | 81 | 115 | 195.970481(13) | 1.84(3) h | β+ | 196Hg | 2− | ||||||||||||
| 196mTl | 394.2(5) keV | 1.41(2) h | β+ (95.5%) | 196Hg | (7+) | ||||||||||||||
| IT (4.5%) | 196Tl | ||||||||||||||||||
| 197Tl | 81 | 116 | 196.969575(18) | 2.84(4) h | β+ | 197Hg | 1/2+ | ||||||||||||
| 197mTl | 608.22(8) keV | 540(10) ms | IT | 197Tl | 9/2− | ||||||||||||||
| 198Tl | 81 | 117 | 197.97048(9) | 5.3(5) h | β+ | 198Hg | 2− | ||||||||||||
| 198m1Tl | 543.5(4) keV | 1.87(3) h | β+ (54%) | 198Hg | 7+ | ||||||||||||||
| IT (46%) | 198Tl | ||||||||||||||||||
| 198m2Tl | 687.2(5) keV | 150(40) ns | (5+) | ||||||||||||||||
| 198m3Tl | 742.3(4) keV | 32.1(10) ms | (10−)# | ||||||||||||||||
| 199Tl | 81 | 118 | 198.96988(3) | 7.42(8) h | β+ | 199Hg | 1/2+ | ||||||||||||
| 199mTl | 749.7(3) keV | 28.4(2) ms | IT | 199Tl | 9/2− | ||||||||||||||
| 200Tl | 81 | 119 | 199.970963(6) | 26.1(1) h | β+ | 200Hg | 2− | ||||||||||||
| 200m1Tl | 753.6(2) keV | 34.3(10) ms | IT | 200Tl | 7+ | ||||||||||||||
| 200m2Tl | 762.0(2) keV | 0.33(5) μs | 5+ | ||||||||||||||||
| 201Tl[n 8] | 81 | 120 | 200.970819(16) | 72.912(17) h | EC | 201Hg | 1/2+ | ||||||||||||
| 201mTl | 919.50(9) keV | 2.035(7) ms | IT | 201Tl | (9/2−) | ||||||||||||||
| 202Tl | 81 | 121 | 201.972106(16) | 12.23(2) d | β+ | 202Hg | 2− | ||||||||||||
| 202mTl | 950.19(10) keV | 572(7) μs | 7+ | ||||||||||||||||
| 203Tl | 81 | 122 | 202.9723442(14) | Observationally Stable[n 9] | 1/2+ | 0.2952(1) | 0.29494–0.29528 | ||||||||||||
| 203mTl | 3400(300) keV | 7.7(5) μs | (25/2+) | ||||||||||||||||
| 204Tl | 81 | 123 | 203.9738635(13) | 3.78(2) y | β− (97.1%) | 204Pb | 2− | ||||||||||||
| EC (2.9%) | 204Hg | ||||||||||||||||||
| 204m1Tl | 1104.0(4) keV | 63(2) μs | (7)+ | ||||||||||||||||
| 204m2Tl | 2500(500) keV | 2.6(2) μs | (12−) | ||||||||||||||||
| 204m3Tl | 3500(500) keV | 1.6(2) μs | (20+) | ||||||||||||||||
| 205Tl[n 10] | 81 | 124 | 204.9744275(14) | Observationally Stable[n 11] | 1/2+ | 0.7048(1) | 0.70472–0.70506 | ||||||||||||
| 205m1Tl | 3290.63(17) keV | 2.6(2) μs | 25/2+ | ||||||||||||||||
| 205m2Tl | 4835.6(15) keV | 235(10) ns | (35/2–) | ||||||||||||||||
| 206Tl | Radium E | 81 | 125 | 205.9761103(15) | 4.200(17) min | β− | 206Pb | 0− | Trace[n 12] | ||||||||||
| 206mTl | 2643.11(19) keV | 3.74(3) min | IT | 206Tl | (12–) | ||||||||||||||
| 207Tl | Actinium C | 81 | 126 | 206.977419(6) | 4.77(2) min | β− | 207Pb | 1/2+ | Trace[n 13] | ||||||||||
| 207mTl | 1348.1(3) keV | 1.33(11) s | IT (99.9%) | 207Tl | 11/2– | ||||||||||||||
| β− (.1%) | 207Pb | ||||||||||||||||||
| 208Tl | Thorium C" | 81 | 127 | 207.9820187(21) | 3.053(4) min | β− | 208Pb | 5+ | Trace[n 14] | ||||||||||
| 209Tl | 81 | 128 | 208.985359(8) | 2.161(7) min | β− | 209Pb | 1/2+ | Trace[n 15] | |||||||||||
| 210Tl | Radium C″ | 81 | 129 | 209.990074(12) | 1.30(3) min | β− (99.991%) | 210Pb | (5+)# | Trace[n 12] | ||||||||||
| β−, n (.009%) | 209Pb | ||||||||||||||||||
| 211Tl | 81 | 130 | 210.993480(50) | 80(16) s | β− (97.8%) | 211Pb | 1/2+ | ||||||||||||
| β−, n (2.2%) | 210Pb | ||||||||||||||||||
| 212Tl | 81 | 131 | 211.998340(220)# | 31(8) s | β− (98.2%) | 212Pb | (5+) | ||||||||||||
| β−, n (1.8%) | 211Pb | ||||||||||||||||||
| 213Tl | 81 | 132 | 213.001915(29) | 24(4) s | β− (92.4%) | 213Pb | 1/2+ | ||||||||||||
| β−, n (7.6%) | 212Pb | ||||||||||||||||||
| 214Tl | 81 | 133 | 214.006940(210)# | 11(2) s | β− (66%) | 214Pb | 5+# | ||||||||||||
| β−, n (34%) | 213Pb | ||||||||||||||||||
| 215Tl | 81 | 134 | 215.010640(320)# | 10(4) s | β− (95.4%) | 215Pb | 1/2+# | ||||||||||||
| β−, n (4.6%) | 214Pb | ||||||||||||||||||
| 216Tl | 81 | 135 | 216.015800(320)# | 6(3) s | β− | 216Pb | 5+# | ||||||||||||
| β−, n (<11.5%) | 215Pb | ||||||||||||||||||
| This table header & footer: | |||||||||||||||||||
- ^ mTl – Excited nuclear isomer.
- ^ ( ) – Uncertainty (1σ) is given in concise form in parentheses after the corresponding last digits.
- ^ # – Atomic mass marked #: value and uncertainty derived not from purely experimental data, but at least partly from trends from the Mass Surface (TMS).
- ^ a b c # – Values marked # are not purely derived from experimental data, but at least partly from trends of neighboring nuclides (TNN).
- ^ Modes of decay:
EC: Electron capture IT: Isomeric transition n: Neutron emission p: Proton emission - ^ Bold symbol as daughter – Daughter product is stable.
- ^ ( ) spin value – Indicates spin with weak assignment arguments.
- ^ Main isotope used in scintigraphy
- ^ Believed to undergo α decay to 199Au
- ^ Final decay product of 4n+1 decay chain (the Neptunium series)
- ^ Believed to undergo α decay to 201Au
- ^ a b Intermediate decay product of 238U
- ^ Intermediate decay product of 235U
- ^ Intermediate decay product of 232Th
- ^ Intermediate decay product of 237Np
Thallium-201
editThallium-201 (201Tl) is a synthetic radioisotope of thallium. It has a half-life of 73 hours and decays by electron capture, emitting X-rays (~70–80 keV), and photons of 135 and 167 keV in 10% total abundance.[17] Thallium-201 is synthesized by the neutron activation of stable thallium in a nuclear reactor,[17][18] or by the 203Tl(p, 3n)201Pb nuclear reaction in cyclotrons, as 201Pb naturally decays to 201Tl afterwards.[19] It is a radiopharmaceutical, as it has good imaging characteristics without excessive patient radiation dose. It is the most popular isotope used for thallium nuclear cardiac stress tests.[20]
References
edit- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ "Standard Atomic Weights: Thallium". CIAAW. 2009.
- ^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (2022-05-04). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ "Thallium Research". doe.gov. Department of Energy. Archived from the original on 2006-12-09. Retrieved 23 March 2018.
- ^ Manual for reactor produced radioisotopes from the International Atomic Energy Agency
- ^ "Bound-state beta decay of highly ionized atoms" (PDF). Archived from the original (PDF) on October 29, 2013. Retrieved June 9, 2013.
- ^ Marcillac, P.; Coron, N.; Dambier, G.; et al. (2003). "Experimental detection of α-particles from the radioactive decay of natural bismuth". Nature. 422 (6934): 876–878. Bibcode:2003Natur.422..876D. doi:10.1038/nature01541. PMID 12712201. S2CID 4415582.
- ^ Half-life, decay mode, nuclear spin, and isotopic composition is sourced in:
Audi, G.; Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S. (2017). "The NUBASE2016 evaluation of nuclear properties" (PDF). Chinese Physics C. 41 (3): 030001. Bibcode:2017ChPhC..41c0001A. doi:10.1088/1674-1137/41/3/030001. - ^ Wang, M.; Audi, G.; Kondev, F. G.; Huang, W. J.; Naimi, S.; Xu, X. (2017). "The AME2016 atomic mass evaluation (II). Tables, graphs, and references" (PDF). Chinese Physics C. 41 (3): 030003-1–030003-442. doi:10.1088/1674-1137/41/3/030003.
- ^ Al-Aqeel, Muneerah Abdullah M. "Decay Spectroscopy of the Thallium Isotopes 176,177Tl". University of Liverpool. ProQuest 2447566201. Retrieved 21 June 2023.
- ^ Poli, G. L.; Davids, C. N.; Woods, P. J.; Seweryniak, D.; Batchelder, J. C.; Brown, L. T.; Bingham, C. R.; Carpenter, M. P.; Conticchio, L. F.; Davinson, T.; DeBoer, J.; Hamada, S.; Henderson, D. J.; Irvine, R. J.; Janssens, R. V. F.; Maier, H. J.; Müller, L.; Soramel, F.; Toth, K. S.; Walters, W. B.; Wauters, J. (1 June 1999). "Proton and $\ensuremath{\alpha}$ radioactivity below the $Z=82$ shell closure". Physical Review C. 59 (6): R2979–R2983. doi:10.1103/PhysRevC.59.R2979. Retrieved 21 June 2023.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (1 March 2021). "The NUBASE2020 evaluation of nuclear physics properties *". Chinese Physics C, High Energy Physics and Nuclear Physics. 45 (3): 030001. Bibcode:2021ChPhC..45c0001K. doi:10.1088/1674-1137/abddae. ISSN 1674-1137. OSTI 1774641.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (1 March 2021). "The NUBASE2020 evaluation of nuclear physics properties *". Chinese Physics C, High Energy Physics and Nuclear Physics. 45 (3): 030001. Bibcode:2021ChPhC..45c0001K. doi:10.1088/1674-1137/abddae. ISSN 1674-1137. OSTI 1774641.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (1 March 2021). "The NUBASE2020 evaluation of nuclear physics properties *". Chinese Physics C, High Energy Physics and Nuclear Physics. 45 (3): 030001. Bibcode:2021ChPhC..45c0001K. doi:10.1088/1674-1137/abddae. ISSN 1674-1137. OSTI 1774641.
- ^ Reich, E. S. (2010). "Mercury serves up a nuclear surprise: a new type of fission". Scientific American. Retrieved 12 May 2011.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (1 March 2021). "The NUBASE2020 evaluation of nuclear physics properties *". Chinese Physics C, High Energy Physics and Nuclear Physics. 45 (3): 030001. Bibcode:2021ChPhC..45c0001K. doi:10.1088/1674-1137/abddae. ISSN 1674-1137. OSTI 1774641.
- ^ a b Audi, Georges; Bersillon, Olivier; Blachot, Jean; Wapstra, Aaldert Hendrik (2003), "The NUBASE evaluation of nuclear and decay properties", Nuclear Physics A, 729: 3–128, Bibcode:2003NuPhA.729....3A, doi:10.1016/j.nuclphysa.2003.11.001
- ^ "Manual for reactor produced radioisotopes" (PDF). International Atomic Energy Agency. 2003. Archived (PDF) from the original on 2011-05-21. Retrieved 2010-05-13.
- ^ Cyclotron Produced Radionuclides: Principles and Practice (PDF). International Atomic Energy Agency. 2008. ISBN 9789201002082. Retrieved 2022-07-01.
- ^ Maddahi, Jamshid; Berman, Daniel (2001). "Detection, Evaluation, and Risk Stratification of Coronary Artery Disease by Thallium-201 Myocardial Perfusion Scintigraphy 155". Cardiac SPECT imaging (2nd ed.). Lippincott Williams & Wilkins. pp. 155–178. ISBN 978-0-7817-2007-6. Archived from the original on 2017-02-22. Retrieved 2016-09-26.
- Isotope masses from:
- Audi, Georges; Bersillon, Olivier; Blachot, Jean; Wapstra, Aaldert Hendrik (2003), "The NUBASE evaluation of nuclear and decay properties", Nuclear Physics A, 729: 3–128, Bibcode:2003NuPhA.729....3A, doi:10.1016/j.nuclphysa.2003.11.001
- Isotopic compositions and standard atomic masses from:
- de Laeter, John Robert; Böhlke, John Karl; De Bièvre, Paul; Hidaka, Hiroshi; Peiser, H. Steffen; Rosman, Kevin J. R.; Taylor, Philip D. P. (2003). "Atomic weights of the elements. Review 2000 (IUPAC Technical Report)". Pure and Applied Chemistry. 75 (6): 683–800. doi:10.1351/pac200375060683.
- Wieser, Michael E. (2006). "Atomic weights of the elements 2005 (IUPAC Technical Report)". Pure and Applied Chemistry. 78 (11): 2051–2066. doi:10.1351/pac200678112051.
- "News & Notices: Standard Atomic Weights Revised". International Union of Pure and Applied Chemistry. 19 October 2005.
- Half-life, spin, and isomer data selected from the following sources.
- Audi, Georges; Bersillon, Olivier; Blachot, Jean; Wapstra, Aaldert Hendrik (2003), "The NUBASE evaluation of nuclear and decay properties", Nuclear Physics A, 729: 3–128, Bibcode:2003NuPhA.729....3A, doi:10.1016/j.nuclphysa.2003.11.001
- National Nuclear Data Center. "NuDat 2.x database". Brookhaven National Laboratory.
- Holden, Norman E. (2004). "11. Table of the Isotopes". In Lide, David R. (ed.). CRC Handbook of Chemistry and Physics (85th ed.). Boca Raton, Florida: CRC Press. ISBN 978-0-8493-0485-9.