Standard electrode potential (data page)
The data below tabulates standard electrode potentials (E°), in volts relative to the standard hydrogen electrode, at:
- Temperature 298.15 K (25.00 °C; 77.00 °F);
- Effective concentration 1 mol/L for each aqueous or amalgamated (mercury-alloyed) species;
- Unit activity for each solvent and pure solid or liquid species; and
- Absolute partial pressure 101.325 kPa (1.00000 atm; 1.01325 bar) for each gaseous reagent — the convention in most literature data but not the current standard state (100 kPa).
The Nernst equation adjusts for general concentrations, pressures, or temperatures.
Simultaneous half-reactions do not in general add voltages, but instead add Gibbs free energy change: the product of the voltage and the number of electrons transferred, typically the Faraday constant. For example, from Fe2+ + 2 e− ⇌ Fe(s) (–0.44 V), the energy to create one neutral atom of Fe(s) from one Fe2+ ion and two electrons is 2 × 0.44 eV = 0.88 eV, or 84 895 J/(mol e−). That value is also the standard formation energy for an Fe2+ ion, since e− and Fe(s) both have zero formation energy.
Data from different sources may cause table inconsistencies. For example:
Table of standard electrode potentials edit
Legend: (s) – solid; (l) – liquid; (g) – gas; (aq) – aqueous (default for all charged species); (Hg) – amalgam; bold – water electrolysis equations.
| Element | Half-reaction | E° / V | Electrons | |||
|---|---|---|---|---|---|---|
| Oxidant | ⇌ | Reductant | ||||
| Sr | Sr+ + e− | ⇌ | Sr(s) | -4.101 | 1 | |
| Ca | Ca+ + e− | ⇌ | Ca(s) | -3.8 | 1 | |
| Th | Th4+ + e− | ⇌ | Th3+ | -3.6 | 1 | |
| Pr | Pr3+ + e− | ⇌ | Pr2+ | -3.1 | 1 | |
| N | 3N 2(g) + 2 H+ + 2 e− | ⇌ | 2HN 3(aq) | -3.09 | 2 | |
| Li | Li+ + e− | ⇌ | Li(s) | -3.0401 | 1 | |
| N | N 2(g) + 4H2O + 2 e− | ⇌ | 2NH 2OH(aq) + 2 OH− | -3.04 | 2 | |
| Cs | Cs+ + e− | ⇌ | Cs(s) | -3.026 | 1 | |
| Ca | Ca(OH) 2 + 2 e− | ⇌ | Ca(s) + 2 OH− | -3.02 | 2 | |
| Er | Er3+ + e− | ⇌ | Er2+ | -3 | 1 | |
| Ba | Ba(OH) 2 + 2 e− | ⇌ | Ba(s) + 2 OH− | -2.99 | 2 | |
| Rb | Rb+ + e− | ⇌ | Rb(s) | -2.98 | 1 | |
| K | K+ + e− | ⇌ | K(s) | -2.931 | 1 | |
| Ba | Ba2+ + 2 e− | ⇌ | Ba(s) | -2.912 | 2 | |
| La | La(OH) 3(s) + 3 e− | ⇌ | La(s) + 3 OH− | -2.9 | 3 | |
| Fr | Fr+ + e− | ⇌ | Fr(s) | -2.9 | 1 | |
| Sr | Sr2+ + 2 e− | ⇌ | Sr(s) | -2.899 | 2 | |
| Sr | Sr(OH) 2 + 2 e− | ⇌ | Sr(s) + 2 OH− | -2.88 | 2 | |
| Ca | Ca2+ + 2 e− | ⇌ | Ca(s) | -2.868 | 2 | |
| Li | Li+ + C 6(s) + e− | ⇌ | LiC 6(s) | -2.84 | 1 | |
| Eu | Eu2+ + 2 e− | ⇌ | Eu(s) | -2.812 | 2 | |
| Ra | Ra2+ + 2 e− | ⇌ | Ra(s) | -2.8 | 2 | |
| Ho | Ho3+ + e− | ⇌ | Ho2+ | -2.8 | 1 | |
| Bk | Bk3+ + e− | ⇌ | Bk2+ | -2.8 | 1 | |
| Yb | Yb2+ + 2 e− | ⇌ | Yb(s) | -2.76 | 2 | |
| Na | Na+ + e− | ⇌ | Na(s) | -2.71 | 1 | |
| Mg | Mg+ + e− | ⇌ | Mg(s) | -2.7 | 1 | |
| Nd | Nd3+ + e− | ⇌ | Nd2+ | -2.7 | 1 | |
| Mg | Mg(OH) 2 + 2 e− | ⇌ | Mg(s) + 2 OH− | -2.69 | 2 | |
| Sm | Sm2+ + 2 e− | ⇌ | Sm(s) | -2.68 | 2 | |
| Be | Be 2O2− 3 + 3H2O + 4 e− | ⇌ | 2Be(s) + 6 OH− | -2.63 | 4 | |
| Pm | Pm3+ + e− | ⇌ | Pm2+ | -2.6 | 1 | |
| Dy | Dy3+ + e− | ⇌ | Dy2+ | -2.6 | 1 | |
| No | No2+ + 2 e− | ⇌ | No | -2.5 | 2 | |
| Hf | HfO(OH) 2 + H2O + 4 e− | ⇌ | Hf(s) + 4 OH− | -2.5 | 4 | |
| Th | Th(OH) 4 + 4 e− | ⇌ | Th(s) + 4 OH− | -2.48 | 4 | |
| Md | Md2+ + 2 e− | ⇌ | Md | -2.4 | 2 | |
| Tm | Tm2+ + 2 e− | ⇌ | Tm(s) | -2.4 | 2 | |
| La | La3+ + 3 e− | ⇌ | La(s) | -2.379 | 3 | |
| Y | Y3+ + 3 e− | ⇌ | Y(s) | -2.372 | 3 | |
| Mg | Mg2+ + 2 e− | ⇌ | Mg(s) | -2.372 | 2 | |
| Sc | ScF3(aq) + 3 H+ + 3 e− | ⇌ | Sc(s) + 3HF(aq) | -2.37 | 3 | |
| Zr | ZrO(OH) 2(s) + H2O + 4 e− | ⇌ | Zr(s) + 4 OH− | -2.36 | 4 | |
| Pr | Pr3+ + 3 e− | ⇌ | Pr(s) | -2.353 | 3 | |
| Ce | Ce3+ + 3 e− | ⇌ | Ce(s) | -2.336 | 3 | |
| Er | Er3+ + 3 e− | ⇌ | Er(s) | -2.331 | 3 | |
| Ho | Ho3+ + 3 e− | ⇌ | Ho(s) | -2.33 | 3 | |
| Al | H 2AlO− 3 + H2O + 3 e− | ⇌ | Al(s) + 4 OH− | -2.33 | 3 | |
| Nd | Nd3+ + 3 e− | ⇌ | Nd(s) | -2.323 | 3 | |
| Tm | Tm3+ + 3 e− | ⇌ | Tm(s) | -2.319 | 3 | |
| Al | Al(OH) 3(s) + 3 e− | ⇌ | Al(s) + 3 OH− | -2.31 | 3 | |
| Sm | Sm3+ + 3 e− | ⇌ | Sm(s) | -2.304 | 3 | |
| Fm | Fm2+ + 2 e− | ⇌ | Fm | -2.3 | 2 | |
| Am | Am3+ + e− | ⇌ | Am2+ | -2.3 | 1 | |
| Dy | Dy3+ + 3 e− | ⇌ | Dy(s) | -2.295 | 3 | |
| Lu | Lu3+ + 3 e− | ⇌ | Lu(s) | -2.28 | 3 | |
| Sc | ScF+ 2 + 2 H+ + 3 e− | ⇌ | Sc(s) + 2HF(l) | -2.28 | 3 | |
| Tb | Tb3+ + 3 e− | ⇌ | Tb(s) | -2.28 | 3 | |
| Gd | Gd3+ + 3 e− | ⇌ | Gd(s) | -2.279 | 3 | |
| H | H 2(g) + 2 e− | ⇌ | 2H− | -2.23 | 2 | |
| Es | Es2+ + 2 e− | ⇌ | Es(s) | -2.23 | 2 | |
| Pm | Pm2+ + 2 e− | ⇌ | Pm(s) | -2.2 | 2 | |
| Tm | Tm3+ + e− | ⇌ | Tm2+ | -2.2 | 1 | |
| Dy | Dy2+ + 2 e− | ⇌ | Dy(s) | -2.2 | 2 | |
| Ac | Ac3+ + 3 e− | ⇌ | Ac(s) | -2.2 | 3 | |
| Yb | Yb3+ + 3 e− | ⇌ | Yb(s) | -2.19 | 3 | |
| Cf | Cf2+ + 2 e− | ⇌ | Cf(s) | -2.12 | 2 | |
| Nd | Nd2+ + 2 e− | ⇌ | Nd(s) | -2.1 | 2 | |
| Ho | Ho2+ + 2 e− | ⇌ | Ho(s) | -2.1 | 2 | |
| Sc | Sc3+ + 3 e− | ⇌ | Sc(s) | -2.077 | 3 | |
| Al | AlF3− 6 + 3 e− | ⇌ | Al(s) + 6F− | -2.069 | 3 | |
| Cm | Cm3+ + 3 e− | ⇌ | Cm(s) | -2.04 | 3 | |
| Pu | Pu3+ + 3 e− | ⇌ | Pu(s) | -2.031 | 3 | |
| Pr | Pr2+ + 2 e− | ⇌ | Pr(s) | -2 | 2 | |
| Er | Er2+ + 2 e− | ⇌ | Er(s) | -2 | 2 | |
| Eu | Eu3+ + 3 e− | ⇌ | Eu(s) | -1.991 | 3 | |
| Lr | Lr3+ + 3 e− | ⇌ | Lr | -1.96 | 3 | |
| Cf | Cf3+ + 3 e− | ⇌ | Cf(s) | -1.94 | 3 | |
| Es | Es3+ + 3 e− | ⇌ | Es(s) | -1.91 | 3 | |
| Pa | Pa4+ + e− | ⇌ | Pa3+ | -1.9 | 1 | |
| Am | Am2+ + 2 e− | ⇌ | Am(s) | -1.9 | 2 | |
| Th | Th4+ + 4 e− | ⇌ | Th(s) | -1.899 | 4 | |
| Fm | Fm3+ + 3 e− | ⇌ | Fm | -1.89 | 3 | |
| N | N2(g) + 2H2O(l) + 4 H+ + 2 e− | ⇌ | 2NH3OH+ | -1.87 | 2 | |
| Np | Np3+ + 3 e− | ⇌ | Np(s) | -1.856 | 3 | |
| Be | Be2+ + 2 e− | ⇌ | Be(s) | -1.847 | 2 | |
| P | H 2PO− 2 + e− | ⇌ | P(s) + 2 OH− | -1.82 | 1 | |
| U | U3+ + 3 e− | ⇌ | U(s) | -1.798 | 3 | |
| Sr | Sr2+ + 2 e− | ⇌ | Sr(Hg) | -1.793 | 2 | |
| B | H 2BO− 3 + H2O + 3 e− | ⇌ | B(s) + 4 OH− | -1.79 | 3 | |
| Th | ThO 2 + 4 H+ + 4 e− | ⇌ | Th(s) + 2H2O | -1.789 | 4 | |
| Hf | HfO2+ + 2 H+ + 4 e− | ⇌ | Hf(s) + H2O | -1.724 | 4 | |
| P | HPO2− 3 + 2H2O + 3 e− | ⇌ | P(s) + 5 OH− | -1.71 | 3 | |
| Si | SiO2− 3 + 3H2O + 4 e− | ⇌ | Si(s) + 6 OH− | -1.697 | 4 | |
| Al | Al3+ + 3 e− | ⇌ | Al(s) | -1.662 | 3 | |
| Ti | Ti2+ + 2 e− | ⇌ | Ti(s) | -1.63 | 2 | |
| Zr | ZrO 2(s) + 4 H+ + 4 e− | ⇌ | Zr(s) + 2H2O | -1.553 | 4 | |
| Zr | Zr4+ + 4 e− | ⇌ | Zr(s) | -1.45 | 4 | |
| Ti | Ti3+ + 3 e− | ⇌ | Ti(s) | -1.37 | 3 | |
| Ti | TiO(s) + 2 H+ + 2 e− | ⇌ | Ti(s) + H2O | -1.31 | 2 | |
| B | B(OH)− 4 + 4H2O(l) + 8 e− | ⇌ | BH− 4 + 8 OH− | -1.24 | 8 | |
| Ga | GaO(OH)− 2 + H2O(l) + 3 e− | ⇌ | Ga(s) + 3 OH− | -1.22 | 3 | |
| Ti | Ti 2O 3(s) + 2 H+ + 2 e− | ⇌ | 2TiO(s) + H2O | -1.23 | 2 | |
| Zn | Zn(OH)2− 4 + 2 e− | ⇌ | Zn(s) + 4 OH− | -1.199 | 2 | |
| Mn | Mn2+ + 2 e− | ⇌ | Mn(s) | -1.185 | 2 | |
| Fe | Fe(CN)4− 6 + 6 H+ + 2 e− | ⇌ | Fe(s) + 6HCN(aq) | -1.16 | 2 | |
| C | C(s) + 3H2O(l) + 2 e− | ⇌ | CH3OH(l) + 2 OH− | -1.148 | 2 | |
| Cr | Cr(CN)3− 6 + e− | ⇌ | Cr(CN)4− 6 | -1.143 | 1 | |
| Te | Te(s) + 2 e− | ⇌ | Te2− | -1.143 | 2 | |
| V | V2+ + 2 e− | ⇌ | V(s) | -1.13 | 2 | |
| Nb | Nb3+ + 3 e− | ⇌ | Nb(s) | -1.099 | 3 | |
| Sn | Sn(s) + 4 H+ + 4 e− | ⇌ | SnH 4(g) | -1.07 | 4 | |
| Po | Po(s) + 2 e− | ⇌ | Po2− | -1.021 | 2 | |
| Cr | [Cr(edta)(H2O)]− + e− | ⇌ | [Cr(edta)(H2O)]2− | -0.99 | 1 | |
| P | 2H3PO4(aq) + 2 H+ + 2 e− | ⇌ | (H2PO3)2(aq) + H2O(l) | -0.933 | 2 | |
| C | CO2− 3 + 3 H+ + 2 e− | ⇌ | HCO− 2 + H2O(l) | -0.93 | 2 | |
| Ti | TiO2+ + 2 H+ + 4 e− | ⇌ | Ti(s) + H2O | -0.93 | 4 | |
| Si | SiO 2(quartz) + 4 H+ + 4 e− | ⇌ | Si(s) + 2H2O | -0.909 | 4 | |
| Cr | Cr2+ + 2 e− | ⇌ | Cr(s) | -0.9 | 2 | |
| B | B(OH) 3(aq) + 3 H+ + 3 e− | ⇌ | B(s) + 3H2O | -0.89 | 3 | |
| Fe | Fe(OH) 2(s) + 2 e− | ⇌ | Fe(s) + 2 OH− | -0.89 | 2 | |
| Fe | Fe 2O 3(s) + 3H2O + 2 e− | ⇌ | 2Fe(OH) 2(s) + 2 OH− | -0.86 | 2 | |
| H | 2H2O + 2 e− | ⇌ | H 2(g) + 2 OH− | -0.8277 | 2 | |
| Bi | Bi(s) + 3 H+ + 3 e− | ⇌ | BiH 3 | -0.8 | 3 | |
| Zn | Zn2+ + 2 e− | ⇌ | Zn(Hg) | -0.7628 | 2 | |
| Zn | Zn2+ + 2 e− | ⇌ | Zn(s) | -0.7618 | 2 | |
| Ta | Ta 2O 5(s) + 10 H+ + 10 e− | ⇌ | 2Ta(s) + 5H2O | -0.75 | 10 | |
| Te | 2Te(s) + 2 e− | ⇌ | Te2− 2 | -0.74 | 2 | |
| Ni | Ni(OH) 2(s) + 2 e− | ⇌ | Ni(s) + 2 OH− | -0.72 | 2 | |
| Nb | Nb2O5(s) + 10 H+ + 10 e− | ⇌ | 2Nb(s) + 5H2O(l) | -0.7 | 10 | |
| Ag | Ag 2S(s) + 2 e− | ⇌ | 2Ag(s) + S2− (aq) | -0.69 | 2 | |
| Te | Te2− 2 + 4 H+ + 2 e− | ⇌ | 2H2Te(g) | -0.64 | 2 | |
| Sb | Sb(OH)− 4 + 3 e− | ⇌ | Sb(s) + 4 OH− | -0.639 | 3 | |
| Au | [Au(CN) 2]− + e− | ⇌ | Au(s) + 2CN− | -0.6 | 1 | |
| Ta | Ta3+ + 3 e− | ⇌ | Ta(s) | -0.6 | 3 | |
| Pb | PbO(s) + H2O + 2 e− | ⇌ | Pb(s) + 2 OH− | -0.580 | 2 | |
| Ti | 2TiO 2(s) + 2 H+ + 2 e− | ⇌ | Ti 2O 3(s) + H2O | -0.56 | 2 | |
| Ga | Ga3+ + 3 e− | ⇌ | Ga(s) | -0.549 | 3 | |
| U | U4+ + e− | ⇌ | U3+ | -0.52 | 1 | |
| P | H 3PO 2(aq) + H+ + e− | ⇌ | P(white)[note 1] + 2H2O | -0.508 | 1 | |
| P | H 3PO 3(aq) + 2 H+ + 2 e− | ⇌ | H 3PO 2(aq) + H2O | -0.499 | 2 | |
| Ni | NiO 2(s) + 2H2O + 2 e− | ⇌ | Ni(OH) 2(s) + 2 OH− | -0.49 | 2 | |
| Sb | Sb(OH)− 6 + 2 e− | ⇌ | Sb(OH)− 4 + 2 OH− | -0.465 | 2 | |
| P | H 3PO 3(aq) + 3 H+ + 3 e− | ⇌ | P(red)[note 1] + 3H2O | -0.454 | 3 | |
| Bi | Bi2O3(s) + 3H2O(l) + 6 e− | ⇌ | Bi(s) + 6 OH− | -0.452 | 6 | |
| Ta | TaF2− 7 + 7 H+ + 5 e− | ⇌ | Ta(s) + 7HF(l) | -0.45 | 5 | |
| In | In3+ + 2 e− | ⇌ | In+ | -0.444 | 2 | |
| Cu | Cu(CN)− 2 + e− | ⇌ | Cu(s) + 2CN− | -0.44 | 1 | |
| Fe | Fe2+ + 2 e− | ⇌ | Fe(s) | -0.44 | 2 | |
| C | 2CO 2(g) + 2 H+ + 2 e− | ⇌ | HOOCCOOH(aq) | -0.43 | 2 | |
| Cr | Cr3+ + e− | ⇌ | Cr2+ | -0.407 | 1 | |
| Cd | Cd2+ + 2 e− | ⇌ | Cd(s) | -0.4 | 2 | |
| Ti | Ti3+ + e− | ⇌ | Ti2+ | -0.37 | 1 | |
| Cu | Cu 2O(s) + H2O + 2 e− | ⇌ | 2Cu(s) + 2 OH− | -0.36 | 2 | |
| Pb | PbSO 4(s) + 2 e− | ⇌ | Pb(s) + SO2− 4 | -0.3588 | 2 | |
| Pb | PbSO 4(s) + 2 e− | ⇌ | Pb(Hg) + SO2− 4 | -0.3505 | 2 | |
| Eu | Eu3+ + e− | ⇌ | Eu2+ | -0.35 | 1 | |
| In | In3+ + 3 e− | ⇌ | In(s) | -0.34 | 3 | |
| Tl | Tl+ + e− | ⇌ | Tl(s) | -0.34 | 1 | |
| Ge | Ge(s) + 4 H+ + 4 e− | ⇌ | GeH 4(g) | -0.29 | 4 | |
| Co | Co2+ + 2 e− | ⇌ | Co(s) | -0.28 | 2 | |
| P | H 3PO 4(aq) + 2 H+ + 2 e− | ⇌ | H 3PO 3(aq) + H2O | -0.276 | 2 | |
| N | N2(g) + 8 H+ + 6 e− | ⇌ | 2NH+ 4 | -0.27 | 6 | |
| V | V3+ + e− | ⇌ | V2+ | -0.26 | 1 | |
| Ni | Ni2+ + 2 e− | ⇌ | Ni(s) | -0.257 | 2 | |
| S | 2HSO− 4 + 2 H+ + 2 e− | ⇌ | S2O2− 6 + 2H2O(l) | -0.253 | 2 | |
| As | As(s) + 3 H+ + 3 e− | ⇌ | AsH 3(g) | -0.23 | 3 | |
| N | N2(g) + 5 H+ + 4 e− | ⇌ | N2H+ 5 | -0.23 | 4 | |
| Ga | Ga+ + e− | ⇌ | Ga(s) | -0.2 | 1 | |
| Ag | AgI(s) + e− | ⇌ | Ag(s) + I− | -0.15224 | 1 | |
| Ge | GeO2(s) + 4 H+ + 4 e− | ⇌ | Ge(s) + 2H2O(l) | -0.15 | 4 | |
| Mo | MoO 2(s) + 4 H+ + 4 e− | ⇌ | Mo(s) + 2H2O | -0.15 | 4 | |
| Si | Si(s) + 4 H+ + 4 e− | ⇌ | SiH 4(g) | -0.14 | 4 | |
| Sn | Sn2+ + 2 e− | ⇌ | Sn(s) | -0.13 | 2 | |
| O | O 2(g) + H+ + e− | ⇌ | HO• 2(aq) | -0.13 | 1 | |
| In | In+ + e− | ⇌ | In(s) | -0.126 | 1 | |
| Pb | Pb2+ + 2 e− | ⇌ | Pb(s) | -0.126 | 2 | |
| W | WO 2(s) + 4 H+ + 4 e− | ⇌ | W(s) + 2H2O | -0.12 | 4 | |
| Ge | GeO 2(s) + 2 H+ + 2 e− | ⇌ | GeO(s) + H2O | -0.118 | 2 | |
| P | P(red) + 3 H+ + 3 e− | ⇌ | PH 3(g) | -0.111 | 3 | |
| C | CO 2(g) + 2 H+ + 2 e− | ⇌ | HCOOH(aq) | -0.11 | 2 | |
| Se | Se(s) + 2 H+ + 2 e− | ⇌ | H 2Se(g) | -0.11 | 2 | |
| C | CO 2(g) + 2 H+ + 2 e− | ⇌ | CO(g) + H2O | -0.11 | 2 | |
| Sn | α-SnO(s) + 2 H+ + 2 e− | ⇌ | Sn(s) + H2O | -0.104 | 2 | |
| Cu | Cu(NH 3)+ 2 + e− | ⇌ | Cu(s) + 2NH 3(aq) | -0.1 | 1 | |
| Nb | Nb2O5(s) + 10 H+ + 4 e− | ⇌ | 2Nb3+ + 5H2O(l) | -0.1 | 4 | |
| W | WO 3(aq) + 6 H+ + 6 e− | ⇌ | W(s) + 3H2O | -0.09 | 6 | |
| Sn | SnO 2(s) + 2 H+ + 2 e− | ⇌ | α-SnO(s) + H2O | -0.088 | 2 | |
| Fe | Fe 3O 4(s) + 8 H+ + 8 e− | ⇌ | 3Fe(s) + 4H2O | -0.085 | 8 | |
| V | VOH2+ + H+ + e− | ⇌ | V2+ + H2O(l) | -0.082 | 1 | |
| P | P(white) + 3 H+ + 3 e− | ⇌ | PH 3(g) | -0.063 | 3 | |
| N | N2O(g) + H2O(l) + 6 H+ + 4 e− | ⇌ | 2NH3OH+ | -0.05 | 4 | |
| Fe | Fe3+ + 3 e− | ⇌ | Fe(s) | -0.04 | 3 | |
| C | HCOOH(aq) + 2 H+ + 2 e− | ⇌ | HCHO(aq) + H2O | -0.034 | 2 | |
| H | 2 H+ + 2 e− | ⇌ | H 2(g) | 0 | 2 | |
| Ag | AgBr(s) + e− | ⇌ | Ag(s) + Br− | 0.07133 | 1 | |
| S | S 4O2− 6 + 2 e− | ⇌ | 2S 2O2− 3 | 0.08 | 2 | |
| N | N 2(g) + 2H2O + 6 H+ + 6 e− | ⇌ | 2NH 4OH(aq) | 0.092 | 6 | |
| Hg | HgO(s) + H2O + 2 e− | ⇌ | Hg(l) + 2 OH− | 0.0977 | 2 | |
| Cu | Cu(NH 3)2+ 4 + e− | ⇌ | Cu(NH 3)+ 2 + 2NH 3(aq) | 0.1 | 1 | |
| Ru | Ru(NH 3)3+ 6 + e− | ⇌ | Ru(NH 3)2+ 6 | 0.1 | 1 | |
| N | N 2H 4(aq) + 4H2O + 2 e− | ⇌ | 2NH+ 4 + 4 OH− | 0.11 | 2 | |
| Mo | H 2MoO 4(aq) + 6 H+ + 6 e− | ⇌ | Mo(s) + 4H2O | 0.11 | 6 | |
| Ge | Ge4+ + 4 e− | ⇌ | Ge(s) | 0.12 | 4 | |
| C | C(s) + 4 H+ + 4 e− | ⇌ | CH 4(g) | 0.13 | 4 | |
| C | HCHO(aq) + 2 H+ + 2 e− | ⇌ | CH 3OH(aq) | 0.13 | 2 | |
| S | S(s) + 2 H+ + 2 e− | ⇌ | H 2S(g) | 0.144 | 2 | |
| Sb | Sb2O3(s) + 6 H+ + 6 e− | ⇌ | 2Sb(s) + 3H2O | 0.15 | 6 | [6]: 789 |
| Sn | Sn4+ + 2 e− | ⇌ | Sn2+ | 0.151 | 2 | |
| S | HSO− 4 + 3 H+ + 2 e− | ⇌ | SO 2(aq) + 2H2O | 0.158 | 2 | |
| Cu | Cu2+ + e− | ⇌ | Cu+ | 0.159 | 1 | |
| U | UO2+ 2 + e− | ⇌ | UO+ 2 | 0.163 | 1 | |
| S | SO2− 4 + 4 H+ + 2 e− | ⇌ | SO 2(aq) + 2H2O | 0.17 | 2 | |
| Ti | TiO2+ + 2 H+ + e− | ⇌ | Ti3+ + H2O | 0.19 | 1 | |
| Sb | SbO+ + 2 H+ + 3 e− | ⇌ | Sb(s) + H2O | 0.2 | 3 | |
| Fe | 3Fe 2O 3(s) + 2 H+ + 2 e− | ⇌ | 2Fe 3O 4(s) + H2O | 0.22 | 2 | |
| Ag | AgCl(s) + e− | ⇌ | Ag(s) + Cl− | 0.22233 | 1 | |
| As | H 3AsO 3(aq) + 3 H+ + 3 e− | ⇌ | As(s) + 3H2O | 0.24 | 3 | |
| Ru | Ru3+ (aq) + e− | ⇌ | Ru2+ (aq) | 0.249 | 1 | |
| Pb | PbO2(s) + H2O + 2 e− | ⇌ | α-PbO(s) + 2 OH− | 0.254 | 2 | |
| Ge | GeO(s) + 2 H+ + 2 e− | ⇌ | Ge(s) + H2O | 0.26 | 2 | |
| Hg | Hg2Cl2(s) + 2 e− | ⇌ | 2Hg(l) + 2Cl− | 0.27 | 2 | |
| U | UO+ 2 + 4 H+ + e− | ⇌ | U4+ + 2H2O | 0.273 | 1 | |
| Re | Re3+ + 3 e− | ⇌ | Re(s) | 0.300 | 3 | |
| At | At + e− | ⇌ | At− | 0.3 | 1 | |
| Bi | Bi3+ + 3 e− | ⇌ | Bi(s) | 0.308 | 3 | |
| C | 2HCNO + 2 H+ + 2 e− | ⇌ | (CN)2 + 2H2O | 0.330 | 2 | |
| Cu | Cu2+ + 2 e− | ⇌ | Cu(s) | 0.337 | 2 | |
| V | VO2+ + 2 H+ + e− | ⇌ | V3+ + H2O | 0.337 | 1 | |
| Sb | Sb2O4(s) + 2 H+ + 2 e− | ⇌ | Sb2O3(s) + H2O(l) | 0.342 | 2 | |
| At | At+ + 2 e− | ⇌ | At- | 0.36 | 2 | |
| Fe | [Fe(CN) 6]3− + e− | ⇌ | [Fe(CN) 6]4− | 0.3704 | 1 | |
| C | (CN)2 + 2 H+ + 2 e− | ⇌ | 2HCN | 0.373 | 2 | |
| P | (H2PO3)2(aq) + 2 H+ + 2 e− | ⇌ | 2H3PO3 | 0.38 | 2 | |
| S | 2SO2(aq) + 2 H+ + 2 e− | ⇌ | S2O2− 3 + H2O(l) | 0.4 | 2 | |
| O | O 2(g) + 2H2O + 4 e− | ⇌ | 4 OH−(aq) | 0.401 | 4 | |
| Mo | H 2MoO 4 + 6 H+ + 3 e− | ⇌ | Mo3+ + 4H2O | 0.43 | 3 | |
| Ru | Ru2+ (aq) + 2 e− | ⇌ | Ru | 0.455 | 2 | |
| V | VO(OH)+ + 2 H+ + e− | ⇌ | VOH2+ + H2O(l) | 0.481 | 1 | |
| C | CH 3OH(aq) + 2 H+ + 2 e− | ⇌ | CH 4(g) + H2O | 0.5 | 2 | |
| S | SO 2(aq) + 4 H+ + 4 e− | ⇌ | S(s) + 2H2O | 0.5 | 4 | |
| S | 4SO 2(aq) + 4 H+ + 8 e− | ⇌ | S4O2− 6 + 2H2O(l) | 0.51 | 8 | |
| Cu | Cu+ + e− | ⇌ | Cu(s) | 0.52 | 1 | |
| C | CO(g) + 2 H+ + 2 e− | ⇌ | C(s) + H2O | 0.52 | 2 | |
| I | I− 3 + 2 e− | ⇌ | 3I− | 0.53 | 2 | |
| Te | TeO2(s) + 4 H+ + 4 e− | ⇌ | Te(s) + 2H2O(l) | 0.53 | 4 | |
| Cu | Cu2+ + Cl− + e− | ⇌ | CuCl(s) | 0.54 | 1 | |
| I | I 2(s) + 2 e− | ⇌ | 2I− | 0.54 | 2 | |
| Au | [AuI 4]− + 3 e− | ⇌ | Au(s) + 4I− | 0.56 | 3 | |
| As | H 3AsO 4(aq) + 2 H+ + 2 e− | ⇌ | H 3AsO 3(aq) + H2O | 0.56 | 2 | |
| S | S2O2− 6 + 4 H+ + 2 e− | ⇌ | 2H2SO3 | 0.569 | 2 | |
| Au | [AuI 2]− + e− | ⇌ | Au(s) + 2I− | 0.58 | 1 | |
| Mn | MnO− 4 + 2H2O + 3 e− | ⇌ | MnO 2(s) + 4 OH− | 0.595 | 3 | |
| S | S 2O2− 3 + 6 H+ + 4 e− | ⇌ | 2S(s) + 3H2O | 0.6 | 4 | |
| Fe | Fc+ + e− | ⇌ | Fc(s) | 0.63 | 1 | |
| Mo | H 2MoO 4(aq) + 2 H+ + 2 e− | ⇌ | MoO 2(s) + 2H2O | 0.65 | 2 | |
| N | HN3(aq) + 11 H+ + 8 e− | ⇌ | 3NH+ 4 | 0.69 | 8 | |
| O | O 2(g) + 2 H+ + 2 e− | ⇌ | H 2O 2(aq) | 0.695 | 2 | |
| Sb | Sb2O5(s) + 4 H+ + 4 e− | ⇌ | Sb2O3(s) + 2H2O | 0.699 | 4 | |
| C | 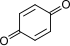 + 2 H+ + 2 e− + 2 H+ + 2 e− | ⇌ | 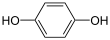 | 0.6992 | 2 | |
| V | H2V10O4− 28 + 24 H+ + 10 e− | ⇌ | 10VO(OH)+ + 8H2O(l) | 0.723 | 10 | |
| Pt | PtCl2− 6 + 2 e− | ⇌ | PtCl2− 4 + 2Cl− | 0.726 | 2 | |
| Fe | Fe 2O 3(s) + 6 H+ + 2 e− | ⇌ | 2Fe2+ + 3H2O | 0.728 | 2 | |
| Se | H 2SeO 3(aq) + 4 H+ + 4 e− | ⇌ | Se(s) + 3H2O | 0.74 | 4 | |
| At | AtO+ + 2 H+ + 2 e− | ⇌ | At+ + H2O | 0.74 | 2 | |
| Tl | Tl3+ + 3 e− | ⇌ | Tl(s) | 0.741 | 3 | |
| No | No3+ + e− | ⇌ | No2+ | 0.75 | 1 | |
| Pt | PtCl2− 4 + 2 e− | ⇌ | Pt(s) + 4Cl− | 0.758 | 2 | |
| Br | BrO− + H2O(l) + 2 e− | ⇌ | Br− + 2 OH− | 0.76 | 2 | |
| Po | Po4+ + 4 e− | ⇌ | Po | 0.76 | 4 | |
| S | (SCN)2 + 2 e− | ⇌ | 2SCN- | 0.769 | 2 | |
| Fe | Fe3+ + e− | ⇌ | Fe2+ | 0.771 | 1 | |
| Hg | Hg2+ 2 + 2 e− | ⇌ | 2Hg(l) | 0.7973 | 2 | |
| Ag | Ag+ + e− | ⇌ | Ag(s) | 0.7996 | 1 | |
| N | 2NO− 3(aq) + 4 H+ + 2 e− | ⇌ | N 2O 4(g) + 2H2O | 0.803 | 2 | |
| Fe | 2FeO2− 4 + 5H2O + 6 e− | ⇌ | Fe 2O 3(s) + 10 OH− | 0.81 | 6 | |
| Au | [AuBr 4]− + 3 e− | ⇌ | Au(s) + 4Br− | 0.85 | 3 | |
| Hg | Hg2+ + 2 e− | ⇌ | Hg(l) | 0.85 | 2 | |
| Ir | [IrCl 6]2− + e− | ⇌ | [IrCl 6]3− | 0.87 | 1 | |
| Mn | MnO− 4 + H+ + e− | ⇌ | HMnO− 4 | 0.9 | 1 | |
| Po | Po4+ + 2 e− | ⇌ | Po2+ | 0.9 | 2 | |
| Hg | 2Hg2+ + 2 e− | ⇌ | Hg2+ 2 | 0.91 | 2 | |
| Pd | Pd2+ + 2 e− | ⇌ | Pd(s) | 0.915 | 2 | |
| Au | [AuCl 4]− + 3 e− | ⇌ | Au(s) + 4Cl− | 0.93 | 3 | |
| N | NO− 3 + 3 H+ + 2 e− | ⇌ | HNO2(aq) | 0.94 | 2 | |
| Mn | MnO 2(s) + 4 H+ + e− | ⇌ | Mn3+ + 2H2O | 0.95 | 1 | |
| N | NO− 3(aq) + 4 H+ + 3 e− | ⇌ | NO(g) + 2H2O(l) | 0.958 | 3 | |
| Au | [AuBr 2]− + e− | ⇌ | Au(s) + 2Br− | 0.96 | 1 | |
| Fe | Fe 3O 4(s) + 8 H+ + 2 e− | ⇌ | 3Fe2+ + 4H2O | 0.98 | 2 | |
| Xe | [HXeO 6]3− + 2H2O + 2 e− | ⇌ | [HXeO 4]− + 4 OH− | 0.99 | 2 | |
| N | HNO2(aq) + H+ + e− | ⇌ | NO(g) + H2O(l) | 0.996 | 1 | |
| At | HAtO + H+ + e− | ⇌ | At + H2O | 1.0 | 1 | |
| V | [VO 2]+ (aq) + 2 H+ + e− | ⇌ | [VO]2+ (aq) + H2O | 1 | 1 | |
| Te | H 6TeO 6(aq) + 2 H+ + 2 e− | ⇌ | TeO 2(s) + 4H2O | 1.02 | 2 | |
| N | NO2(g) + 2 H+ + 2 e− | ⇌ | NO(g) + H2O(l) | 1.03 | 2 | |
| Br | Br− 3 + 2 e− | ⇌ | 3Br− | 1.05 | 2 | |
| Sb | Sb2O5(s) + 2 H+ + 2 e− | ⇌ | Sb2O4(s) + H2O(l) | 1.055 | 2 | |
| I | ICl− 2 + e− | ⇌ | 2Cl− + I(s) | 1.06 | 1 | |
| Br | Br 2(l) + 2 e− | ⇌ | 2Br− | 1.066 | 2 | |
| N | N2O4(g) + 2 H+ + 2 e− | ⇌ | 2HNO2 | 1.07 | 2 | |
| Br | Br 2(aq) + 2 e− | ⇌ | 2Br− | 1.0873 | 2 | |
| Ru | RuO 2 + 4 H+ + 2 e− | ⇌ | Ru2+ (aq) + 2H2O | 1.120 | 2 | |
| Cu | Cu2+ + 2CN− + e− | ⇌ | Cu(CN)− 2 | 1.12 | 1 | |
| I | IO− 3 + 5 H+ + 4 e− | ⇌ | HIO(aq) + 2H2O | 1.13 | 4 | |
| O | H2O2(aq) + H+ + e− | ⇌ | H2O(l) + HO• | 1.14 | 1 | |
| Au | [AuCl 2]− + e− | ⇌ | Au(s) + 2Cl− | 1.15 | 1 | |
| Se | HSeO− 4 + 3 H+ + 2 e− | ⇌ | H 2SeO 3(aq) + H2O | 1.15 | 2 | |
| Ag | Ag 2O(s) + 2 H+ + 2 e− | ⇌ | 2Ag(s) + H2O | 1.17 | 2 | |
| Cl | ClO− 3 + 2 H+ + e− | ⇌ | ClO 2(g) + H2O | 1.175 | 1 | |
| Xe | [HXeO 6]3− + 5H2O + 8 e− | ⇌ | Xe(g) + 11 OH− | 1.18 | 8 | |
| Pt | Pt2+ + 2 e− | ⇌ | Pt(s) | 1.188 | 2 | |
| Cl | ClO 2(g) + H+ + e− | ⇌ | HClO 2(aq) | 1.19 | 1 | |
| I | 2IO− 3 + 12 H+ + 10 e− | ⇌ | I 2(s) + 6H2O | 1.2 | 10 | |
| Mn | MnO 2(s) + 4 H+ + 2 e− | ⇌ | Mn2+ + 2H2O | 1.224 | 2 | |
| O | O 2(g) + 4 H+ + 4 e− | ⇌ | 2H2O | 1.229 | 4 | |
| N | N2H+ 5 + 3 H+ + 2 e− | ⇌ | 2NH+ 4 | 1.28 | 2 | |
| Cl | ClO− 4 + 2 H+ + 2 e− | ⇌ | ClO− 3 + H2O | 1.23 | 2 | |
| Ru | [Ru(bipy) 3]3+ + e− | ⇌ | [Ru(bipy) 3]2+ | 1.24 | 1 | |
| Xe | [HXeO 4]− + 3H2O + 6 e− | ⇌ | Xe(g) + 7 OH− | 1.24 | 6 | |
| N | 2NO− 3 + 12 H+ + 10 e− | ⇌ | N2(g) + 6H2O(l) | 1.25 | 10 | |
| Tl | Tl3+ + 2 e− | ⇌ | Tl+ | 1.25 | 2 | |
| N | 2HNO2(aq) + 4 H+ + 4 e− | ⇌ | N2O(g) + 3H2O(l) | 1.297 | 4 | |
| Cr | Cr 2O2− 7 + 14 H+ + 6 e− | ⇌ | 2Cr3+ + 7H2O | 1.38 | 6 | |
| N | NH3OH+ + 2 H+ + 2 e− | ⇌ | NH+ 4 + H2O(l) | 1.35 | 2 | |
| Cl | Cl 2(g) + 2 e− | ⇌ | 2Cl− | 1.36 | 2 | |
| Ru | RuO− 4(aq) + 8 H+ + 5 e− | ⇌ | Ru2+ (aq) + 4H2O | 1.368 | 5 | |
| Ru | RuO 4 + 4 H+ + 4 e− | ⇌ | RuO 2 + 2H2O | 1.387 | 4 | |
| Co | CoO 2(s) + 4 H+ + e− | ⇌ | Co3+ + 2H2O | 1.42 | 1 | |
| N | 2NH 3OH+ + H+ + 2 e− | ⇌ | N 2H+ 5 + 2H2O | 1.42 | 2 | |
| I | 2HIO(aq) + 2 H+ + 2 e− | ⇌ | I 2(s) + 2H2O | 1.44 | 2 | |
| Br | BrO− 3 + 5 H+ + 4 e− | ⇌ | HBrO(aq) + 2H2O | 1.447 | 4 | |
| Pb | β-PbO 2(s) + 4 H+ + 2 e− | ⇌ | Pb2+ + 2H2O | 1.46 | 2 | |
| Pb | α-PbO 2(s) + 4 H+ + 2 e− | ⇌ | Pb2+ + 2H2O | 1.468 | 2 | |
| Br | 2BrO− 3 + 12 H+ + 10 e− | ⇌ | Br 2(l) + 6H2O | 1.48 | 10 | |
| At | HAtO3 + 4 H+ + 4 e− | ⇌ | HAtO + 2H2O | 1.5 | 4 | |
| Mn | MnO− 4 + 8 H+ + 5 e− | ⇌ | Mn2+ + 4H2O | 1.51 | 5 | |
| O | HO• 2 + H+ + e− | ⇌ | H 2O 2(aq) | 1.51 | 1 | |
| Au | Au3+ + 3 e− | ⇌ | Au(s) | 1.52 | 3 | |
| Ru | RuO2− 4(aq) + 8 H+ + 4 e− | ⇌ | Ru2+ (aq) + 4H2O | 1.563 | 4 | |
| N | 2NO(g) + 2 H+ + 2 e− | ⇌ | N2O(g) + H2O(l) | 1.59 | 2 | |
| Ni | NiO 2(s) + 2 H+ + 2 e− | ⇌ | Ni2+ + 2 OH− | 1.59 | 2 | |
| Ce | Ce4+ + e− | ⇌ | Ce3+ | 1.61 | 1 | |
| Cl | 2HClO(aq) + 2 H+ + 2 e− | ⇌ | Cl 2(g) + 2H2O | 1.63 | 2 | |
| I | IO− 4 + 2 H+ + 2 e− | ⇌ | IO− 3 + H2O | 1.64 | 2 | |
| Ag | Ag 2O 3(s) + 6 H+ + 4 e− | ⇌ | 2Ag+ + 3H2O | 1.67 | 4 | |
| Cl | HClO 2(aq) + 2 H+ + 2 e− | ⇌ | HClO(aq) + H2O | 1.67 | 2 | |
| Pb | Pb4+ + 2 e− | ⇌ | Pb2+ | 1.69 | 2 | |
| Mn | MnO− 4 + 4 H+ + 3 e− | ⇌ | MnO 2(s) + 2H2O | 1.7 | 3 | |
| Br | BrO− 4 + 2 H+ + 2 e− | ⇌ | BrO− 3 + H2O | 1.74 | 2 | |
| Ag | AgO(s) + 2 H+ + e− | ⇌ | Ag+ + H2O | 1.77 | 1 | |
| N | N2O(g) + 2 H+ + 2 e− | ⇌ | N2(g) + H2O(l) | 1.77 | 2 | [6]: 789 |
| O | H 2O 2(aq) + 2 H+ + 2 e− | ⇌ | 2H2O | 1.78 | 2 | |
| Au | Au+ + e− | ⇌ | Au(s) | 1.83 | 1 | |
| Co | Co3+ + e− | ⇌ | Co2+ | 1.92 | 1 | |
| Ag | Ag2+ + e− | ⇌ | Ag+ | 1.98 | 1 | |
| O | S 2O2− 8 + 2 e− | ⇌ | 2SO2− 4 | 2.01 | 2 | |
| O | O 3(g) + 2 H+ + 2 e− | ⇌ | O 2(g) + H2O | 2.075 | 2 | |
| Mn | HMnO− 4 + 3 H+ + 2 e− | ⇌ | MnO 2(s) + 2H2O | 2.09 | 2 | |
| Xe | XeO 3(aq) + 6 H+ + 6 e− | ⇌ | Xe(g) + 3H2O | 2.12 | 6 | |
| Xe | H 4XeO 6(aq) + 8 H+ + 8 e− | ⇌ | Xe(g) + 6H2O | 2.18 | 8 | |
| Fe | FeO2− 4 + 8 H+ + 3 e− | ⇌ | Fe3+ + 4H2O | 2.2 | 3 | |
| Xe | XeF 2(aq) + 2 H+ + 2 e− | ⇌ | Xe(g) + 2HF(aq) | 2.32 | 2 | |
| O | HO• + H+ + e− | ⇌ | H2O(l) | 2.38 | 1 | |
| Xe | H 4XeO 6(aq) + 2 H+ + 2 e− | ⇌ | XeO 3(aq) + 3H2O | 2.42 | 2 | |
| F | F 2(g) + 2 e− | ⇌ | 2F− | 2.87 | 2 | |
| Cm | Cm4+ + e– | ⇌ | Cm3+ | 3.0 | 1 | |
| F | F 2(g) + 2 H+ + 2 e− | ⇌ | 2HF(aq) | 3.077 | 2 | |
| Tb | Tb4+ + e– | ⇌ | Tb3+ | 3.1 | 1 | |
| Pr | Pr4+ + e– | ⇌ | Pr3+ | 3.2 | 1 | |
| Kr | KrF 2(aq) + 2 e− | ⇌ | Kr(g) + 2F− (aq) | 3.27 | 2 | |
See also edit
Notes edit
References edit
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as at au av aw ax ay az ba bb bc bd be bf bg bh bi bj bk bl bm bn bo bp bq br bs bt bu bv Lide, David R., ed. (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, FL: CRC Press. ISBN 0-8493-0487-3.
- ^ Greenwood and Earnshaw, p. 1263
- ^ a b c d e Bratsch, Stephen G. (July 29, 1988) [1 March 1988]. "Standard electrode potentials and temperature coefficients in water at 298.15 K" (PDF). Journal of Physical and Chemical Reference Data. 18 (1). American Institute of Physics (published 1989): 1–21. doi:10.1063/1.555839 – via NIST.
- ^ a b c d Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ a b c d e f g h i j k l m n o p q Vanýsek, Petr (2011). "Electrochemical Series". In Haynes, William M. (ed.). CRC Handbook of Chemistry and Physics (92nd ed.). CRC Press. pp. 5–80–9. ISBN 978-1-4398-5512-6.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as at au av aw ax ay az ba bb bc bd be bf bg bh bi bj bk bl bm bn bo bp bq br bs bt bu bv bw bx by bz ca cb cc cd ce Atkins, Peter; Overton, Tina; Rourke, Jonathan; Weller, Mark; Armstrong, Fraser; Hagerman, Michael (2010). Inorganic Chemistry (5th ed.). New York: W. H. Freeman. ISBN 978-1-42-921820-7.
- ^ a b c d e f g h i j k l m Atkins, Peter W. (1997). Physical Chemistry (6th ed.). W.H. Freeman. ISBN 9780716734659.
- ^ a b c d e f g h i j k l m n o p q r s t u v Petr Vanysek. "Electrochemical series" (PDF). depa.fquim.unam.mx. Archived from the original (PDF) on 2021-09-16.
- ^ David R. Lide, ed., CRC Handbook of Chemistry and Physics, Internet Version 2005, http://www.hbcpnetbase.com Archived 2017-07-24 at the Wayback Machine, CRC Press, Boca Raton, FL, 2005.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab Vanýsek, Petr (2012). "Electrochemical Series". In Haynes, William M. (ed.). Handbook of Chemistry and Physics (93rd ed.). CRC Press. pp. 5–80. ISBN 9781439880494.
- ^ Aylward, Gordon; Findlay, Tristan (2008). SI Chemical Data (6th ed.). Wiley. ISBN 978-0-470-81638-7.
- ^ a b c d e "compounds information". Iron. WebElements Periodic Table of the Elements.
- ^ a b c d e f g h i j k l m n o p q r s t u Bard, Allen J.; Parsons, Roger; Jordan, Joseph (1985). Standard Potentials in Aqueous Solution. CRC Press. ISBN 978-0-8247-7291-8.
- ^ Brown, Susan A.; Brown, Paul L. (2020). "The pH-potential diagram for polonium". The Aqueous Chemistry of Polonium and the Practical Application of its Thermochemistry. Elsevier. doi:10.1016/b978-0-12-819308-2.00004-8. ISBN 978-0-12-819308-2. S2CID 213141476.
- ^ a b c d e f g h i j Bard, A.J.; Faulkner, L.R. (2001). Electrochemical Methods. Fundamentals and Applications (2nd ed.). Wiley. ISBN 9781118312803.
- ^ a b c d e f g h i j k l Lee, J. L. (1983) [1977]. A New Concise Inorganic Chemistry (3rd ed.). London / Wokingham, Berkshire: English Language Book Society & Van Nostrand Reinhold (UK). p. 107. ISBN 0-442-30179-0. OL 4079768W – via the Internet Archive.
- ^ Pourbaix, Marcel (1966). Atlas of Electrochemical Equilibria in Aqueous Solutions. Houston, Texas; Cebelcor, Brussels: NACE International. OCLC 475102548.
- ^ a b c Pang, Suh Cem; Chin, Suk Fun; Anderson, Marc A. (July 2007). "Redox equilibria of iron oxides in aqueous-based magnetite dispersions: Effect of pH and redox potential". J. Colloid Interface Sci. 311 (1): 94–101. Bibcode:2007JCIS..311...94P. doi:10.1016/j.jcis.2007.02.058. PMID 17395194. Retrieved 2017-03-26.
- ^ a b c d e f Greenwood and Earnshaw, p. 1077
- ^ a b c Lavrukhina, Avgusta Konstantinovna; Pozdni︠a︡kov, Aleksandr Aleksandrovich (1970). Analytical chemistry of technetium, promethium, astatine and francium. Ann Arbor: Ann Arbor-Humphrey Science Publishers. p. 237. ISBN 0-250-39923-7. OCLC 186926.
- ^ a b Champion, J.; Alliot, C.; Renault, E.; Mokili, B. M.; Chérel, M.; Galland, N.; Montavon, G. (2009-12-16). "Astatine Standard Redox Potentials and Speciation in Acidic Medium" (PDF). The Journal of Physical Chemistry A. 114 (1). American Chemical Society (ACS): 576–582. doi:10.1021/jp9077008. ISSN 1089-5639. PMID 20014840. S2CID 15738065.
- ^ Rock, Peter A. (February 1966). "The Standard Oxidation Potential of the Ferrocyanide-Ferricyanide Electrode at 25° and the Entropy of Ferrocyanide Ion". The Journal of Physical Chemistry. 70 (2): 576–580. doi:10.1021/j100874a042. ISSN 0022-3654.
- ^ Pavlishchuk, Vitaly V.; Addison, Anthony W. (January 2000). "Conversion constants for redox potentials measured versus different reference electrodes in acetonitrile solutions at 25°C". Inorganica Chimica Acta. 298 (1): 97–102. doi:10.1016/S0020-1693(99)00407-7.
- ^ Toyoshima, A.; Kasamatsu, Y.; Tsukada, K.; Asai, M.; Kitatsuji, Y.; Ishii, Y.; Toume, H.; Nishinaka, I.; Haba, H.; Ooe, K.; Sato, W.; Shinohara, A.; Akiyama, K.; Nagame, Y. (8 July 2009). "Oxidation of element 102, nobelium, with flow electrolytic column chromatography on an atom-at-a-time scale". Journal of the American Chemical Society. 131 (26): 9180–1. doi:10.1021/ja9030038. PMID 19514720.
- ^ Kaufmann, H. P. (1925). "Das freie Rhodan und seine Anwendung in der Maßanalyse. Eine neue Kennzahl der Fette" [Unbound rhodanium and its application to elemental analysis: A new measurement technique for fats]. Archiv der Pharmazie und Berichte der Deutschen Pharmazeutischen Gesellschaft (in German). 263: 675–721 – via HathiTrust.
- ^ a b c d e f g "compounds information". Xenon. WebElements Periodic Table of the Elements.
- ^ a b Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999), Advanced Inorganic Chemistry (6th ed.), New York: Wiley-Interscience, ISBN 0-471-19957-5.
- ^ a b c d e Ghosh, Abhik; Berg, Steffen (2014). Arrow Pushing in Inorganic Chemistry: A logical approach to the chemistry of the main-group elements. Hoboken: Wiley. p. 12. ISBN 978-1-118-17398-5.
- ^ a b c Appelman, Evan H. (1973-04-01). "Nonexistent compounds. Two case histories". Accounts of Chemical Research. 6 (4). American Chemical Society (ACS): 113–117. doi:10.1021/ar50064a001. ISSN 0001-4842.
- ^ Courtney, Arlene. "Oxidation Reduction Chemistry of the Elements". Ch 412 Advanced Inorganic Chemistry: Reading Materials. Western Oregon University.
- ^ Leszczyński, P.J.; Grochala, W. (2013). "Strong Cationic Oxidizers: Thermal Decomposition, Electronic Structure and Magnetism of Their Compounds" (PDF). Acta Chim. Slov. 60 (3): 455–470. PMID 24169699. Archived (PDF) from the original on 2022-10-09.
External links edit
- Chemistry LibreTexts (2021-04-26). "P1: Standard Reduction Potentials by Element". Chemistry LibreTexts. Retrieved 2021-11-30.
- California State University, Northridge (CSUN). "Standard Reduction Potentials" (PDF). csun.edu. Archived (PDF) from the original on 2017-12-15. Retrieved 2021-11-30.
- Wardman, Peter (1989). "Reduction potentials of one-electron couples involving free radicals in aqueous solution" (PDF). srd.nist.gov. Archived (PDF) from the original on 2022-10-09. Retrieved 2021-11-30.
- http://www.jesuitnola.org/upload/clark/Refs/red_pot.htm Archived 2008-07-20 at the Wayback Machine
- https://web.archive.org/web/20150924015049/http://www.fptl.ru/biblioteka/spravo4niki/handbook-of-Chemistry-and-Physics.pdf
- http://hyperphysics.phy-astr.gsu.edu/Hbase/tables/electpot.html#c1

