Sodium-ion batteries (NIBs, SIBs, or Na-ion batteries) are several types of rechargeable batteries, which use sodium ions (Na+) as its charge carriers. In some cases, its working principle and cell construction are similar to those of lithium-ion battery (LIB) types, but it replaces lithium with sodium as the intercalating ion. Sodium belongs to the same group in the periodic table as lithium and thus has similar chemical properties. However, in some cases, such as aqueous batteries, SIBs can be quite different from LIBs.
 A sodium-ion cell (size 18650) | |
| Specific energy | 0.27-0.72 MJ/kg (75–200 W·h/kg) |
|---|---|
| Energy density | 250–375 W·h/L |
| Cycle durability | "thousands"[1] of cycles |
| Nominal cell voltage | 3.0-3.1 V |

SIBs received academic and commercial interest in the 2010s and early 2020s, largely due to lithium's high cost, uneven geographic distribution, and environmentally-damaging extraction process. An obvious advantage of sodium is its natural abundance,[2] particularly in saltwater. Another factor is that cobalt, copper and nickel are not required for many types of sodium-ion batteries, and more abundant iron-based materials (such as NaFeO2 with the Fe3+/Fe4+ redox pair) [3] work well in Na+ batteries. This is because the ionic radius of Na+ (116 pm) is substantially larger than that of Fe2+ and Fe3+ (69–92 pm depending on the spin state), whereas the ionic radius of Li+ is similar (90 pm). Similar ionic radii of lithium and iron result in their mixing in the cathode material during battery cycling, and a resultant loss of cyclable charge. A downside of the larger ionic radius of Na+ is a slower intercalation kinetics of sodium-ion electrode materials.[4]
The development of Na+ batteries started in the 1990s.After three decades of development, NIBs are at a critical moment of commercialization. Several companies such as HiNa and CATL in China, Faradion in the United Kingdom, Tiamat in France, Northvolt in Sweden,[5] and Natron Energy in the US, are close to achieving the commercialization of NIBs, with the aim of employing sodium layered transition metal oxides (NaxTMO2), Prussian white (a Prussian blue analogue[6]) or vanadium phosphate as cathode materials.[7]
Sodium-ion accumulators are operational for fixed electrical grid storage, but vehicles using sodium-ion battery packs are not yet commercially available. However, CATL, the world's biggest lithium-ion battery manufacturer, announced in 2022 the start of mass production of SIBs. In February 2023, the Chinese HiNA Battery Technology Company, Ltd. placed a 140 Wh/kg sodium-ion battery in an electric test car for the first time,[8] and energy storage manufacturer Pylontech obtained the first sodium-ion battery certificate[clarification needed] from TÜV Rheinland.[9]
History edit
Sodium-ion battery development took place in the 1970s and early 1980s. However, by the 1990s, lithium-ion batteries had demonstrated more commercial promise, causing interest in sodium-ion batteries to decline.[10][11] In the early 2010s, sodium-ion batteries experienced a resurgence, driven largely by the increasing cost of lithium-ion battery raw materials.[10]
Operating principle edit
SIB cells consist of a cathode based on a sodium-based material, an anode (not necessarily a sodium-based material) and a liquid electrolyte containing dissociated sodium salts in polar protic or aprotic solvents. During charging, sodium ions move from the cathode to the anode while electrons travel through the external circuit. During discharge, the reverse process occurs.
Materials edit
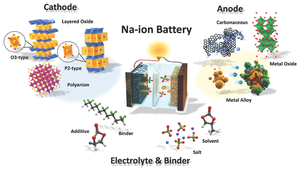
Due to the physical and electrochemical properties of sodium, SIBs require different materials from those used for LIBs.[12]
Anodes edit
Carbons edit
SIBs can use hard carbon, a disordered carbon material consisting of a non-graphitizable, non-crystalline and amorphous carbon. Hard carbon's ability to absorb sodium was discovered in 2000.[13] This anode was shown to deliver 300 mAh/g with a sloping potential profile above ⁓0.15 V vs Na/Na+. It accounts for roughly half of the capacity and a flat potential profile (a potential plateau) below ⁓0.15 V vs Na/Na+. Such capacities are comparable to 300–360 mAh/g of graphite anodes in lithium-ion batteries. The first sodium-ion cell using hard carbon was demonstrated in 2003 and showed a 3.7 V average voltage during discharge.[14] Hard carbon was the preferred choice of Faradion due to its excellent combination of capacity, (lower) working potentials, and cycling stability.[15] Notably, nitrogen-doped hard carbons display even larger specific capacity of 520 mAh/g at 20 mA/g with stability over 1000 cycles.[16]
In 2015, researchers demonstrated that graphite could co-intercalate sodium in ether-based electrolytes. Low capacities around 100 mAh/g were obtained with relatively high working potentials between 0 – 1.2 V vs Na/Na+.[17]
One drawback of carbonaceous materials is that, because their intercalation potentials are fairly negative, they are limited to non-aqueous systems.
Plasma-derived Hard Carbon
Plasma-derived hard carbon is an emerging area for SIB batteries.[18] Plasma-derived hard carbon uplift Coulombic efficiency and specific capacity by 33% and 44%. Spark sintering plasma has shown initial Coulombic efficiency of ~90 % reversible capacity of ~300 mAh/g and rate capacity of 136.6 mAh/g at 5 A/g. The future aspects of plasma methods to perform multi-material doping, in-situ nanoarchitecture fabrications, and challenges around SIB functioning in extreme environments, and the development of real-time robust monitoring and diagnostic tools to make safe, stable, and high-performance SIB with long life. Further, a data-driven manufacturing framework suggests integrating material informatics with experimental protocols for virtual synthesis of hard carbon; estimating material formulations, manufacturing methods, process-property-performance relationship, and limitations before physical manufacturing of high-performance sodium batteries.[18]
Graphene edit
Graphene Janus particles have been used in experimental sodium-ion batteries to increase energy density. One side provides interaction sites while the other provides inter-layer separation. Energy density reached 337 mAh/g.[19]
Carbon arsenide edit
Carbon arsenide (AsC5) mono/bilayer has been explored as an anode material due to high specific gravity (794/596 mAh/g), low expansion (1.2%), and ultra low diffusion barrier (0.16/0.09 eV), indicating rapid charge/discharge cycle capability, during sodium intercalation.[20] After sodium adsorption, a carbon arsenide anode maintains structural stability at 300 K, indicating long cycle life.
Metal alloys edit
Numerous reports described anode materials storing sodium via alloy reaction and/or conversion reaction.[10] Alloying sodium metal brings the benefits of regulating sodium-ion transport and shielding the accumulation of electric field at the tip of sodium dendrites.[21] Wang, et al. reported that a self-regulating alloy interface of nickel antimony (NiSb) was chemically deposited on Na metal during discharge. This thin layer of NiSb regulates the uniform electrochemical plating of Na metal, lowering overpotential and offering dendrite-free plating/stripping of Na metal over 100 h at a high areal capacity of 10 mAh cm−2.[22]
Metals edit
Many metals and semi-metals (Pb, P, Sn, Ge, etc.) form stable alloys with sodium at room temperature. Unfortunately, the formation of such alloys is usually accompanied by a large volume change, which in turn results in the pulverization (crumbling) of the material after a few cycles. For example, with tin sodium forms an alloy Na
15Sn
4, which is equivalent to 847 mAh/g specific capacity, with a resulting enormous volume change up to 420%.[23]
In one study, Li et al. prepared sodium and metallic tin Na
15Sn
4/Na through a spontaneous reaction.[24] This anode could operate at a high temperature of 90 °C (194 °F) in a carbonate solvent at 1 mA cm−2 with 1 mA h cm−2 loading, and the full cell exhibited a stable charge-discharge cycling for 100 cycles at a current density of 2C.[24] (2C means that full charge or discharge was achieved in 0.5 hour). Despite sodium alloy's ability to operate at extreme temperatures and regulate dendritic growth, the severe stress-strain experienced on the material in the course of repeated storage cycles limits cycling stability, especially in large-format cells.
Researchers from Tokyo University of Science achieved 478 mAh/g with nano-sized magnesium particles, announced in December 2020.[25]
Oxides edit
Some sodium titanate phases such as Na2Ti3O7,[26][27][28] or NaTiO2,[29] delivered capacities around 90–180 mAh/g at low working potentials (< 1 V vs Na/Na+), though cycling stability was limited to a few hundred cycles.
Molybdenum disulphide edit
In 2021, researchers from China tried layered structure MoS2 as a new type of anode for sodium-ion batteries. A dissolution-recrystallization process densely assembled carbon layer-coated MoS2 nanosheets onto the surface of polyimide-derived N-doped carbon nanotubes. This kind of C-MoS2/NCNTs anode can store 348 mAh/g at 2 A/g, with a cycling stability of 82% capacity after 400 cycles at 1 A/g.[30] TiS2 is another potential material for SIBs because of its layered structure, but has yet to overcome the problem of capacity fade, since TiS2 suffers from poor electrochemical kinetics and relatively weak structural stability. In 2021, researchers from Ningbo, China employed pre-potassiated TiS2, presenting rate capability of 165.9mAh/g and a cycling stability of 85.3% capacity after 500 cycles.[31]
Other anodes for Na+ edit
Some other materials, such as mercury, electroactive polymers and sodium terephthalate derivatives,[32] have also been demonstrated in laboratories, but did not provoke commercial interest.[15]
Cathodes edit
Oxides edit
Many layered transition metal oxides can reversibly intercalate sodium ions upon reduction. These oxides typically have a higher tap density and a lower electronic resistivity, than other posode materials (such as phosphates). Due to a larger size of the Na+ ion (116 pm) compared to Li+ ion (90 pm), cation mixing between Na+ and first row transition metal ions usually does not occur. Thus, low-cost iron and manganese oxides can be used for Na-ion batteries, whereas Li-ion batteries require the use of more expensive cobalt and nickel oxides. The drawback of the larger size of Na+ ion is its slower intercalation kinetics compared to Li+ ion and the presence of multiple intercalation stages with different voltages and kinetic rates.[4]
A P2-type Na2/3Fe1/2Mn1/2O2 oxide from earth-abundant Fe and Mn resources can reversibly store 190 mAh/g at average discharge voltage of 2.75 V vs Na/Na+ utilising the Fe3+/4+ redox couple – on par or better than commercial lithium-ion cathodes such as LiFePO4 or LiMn2O4.[33] However, its sodium deficient nature lowered energy density. Significant efforts were expended in developing Na-richer oxides. A mixed P3/P2/O3-type Na0.76Mn0.5Ni0.3Fe0.1Mg0.1O2 was demonstrated to deliver 140 mAh/g at an average discharge voltage of 3.2 V vs Na/Na+ in 2015.[34] In particular, the O3-type NaNi1/4Na1/6Mn2/12Ti4/12Sn1/12O2 oxide can deliver 160 mAh/g at average voltage of 3.22 V vs Na/Na+,[35] while a series of doped Ni-based oxides of the stoichiometry NaaNi(1−x−y−z)MnxMgyTizO2 can deliver 157 mAh/g in a sodium-ion "full cell" with a hard carbon anode at average discharge voltage of 3.2 V utilising the Ni2+/4+ redox couple.[36] Such performance in full cell configuration is better or on par with commercial lithium-ion systems. A Na0.67Mn1−xMgxO2 cathode material exhibited a discharge capacity of 175 mAh/g for Na0.67Mn0.95Mg0.05O2. This cathode contained only abundant elements.[37] Copper-substituted Na0.67Ni0.3−xCuxMn0.7O2 cathode materials showed a high reversible capacity with better capacity retention. In contrast to the copper-free Na0.67Ni0.3−xCuxMn0.7O2 electrode, the as-prepared Cu-substituted cathodes deliver better sodium storage. However, cathodes with Cu are more expensive.[38]
Oxoanions edit
Research has also considered cathodes based on oxoanions. Such cathodes offer lower tap density, lowering energy density than oxides. On the other hand, a stronger covalent bonding of the polyanion positively impacts cycle life and safety and increases the cell voltage. Among polyanion-based cathodes, sodium vanadium phosphate[39] and fluorophosphate[40] have demonstrated excellent cycling stability and in the latter, an acceptably high capacity (⁓120 mAh/g) at high average discharge voltages (⁓3.6 V vs Na/Na+).[41] Besides that, sodium manganese silicate has also been demonstrated to deliver a very high capacity (>200 mAh/g) with decent cycling stability.[42] A French startup TIAMAT develops Na+ ion batteries based on a sodium-vanadium-phosphate-fluoride cathode material Na3V2(PO4)2F3, which undergoes two reversible 0.5 e-/V transitions: at 3.2V and at 4.0 V.[43] A startup from Singapore, SgNaPlus is developing and commercialising Na3V2(PO4)2F3 cathode material, which shows very good cycling stability, utilising the non-flammable glyme-based electrolyte.[44]
Prussian blue and analogues edit
Numerous research groups investigated the use of Prussian blue and various Prussian blue analogues (PBAs) as cathodes for Na+-ion batteries. The ideal formula for a discharged material isNa2M[Fe(CN)6], and it corresponds to the theoretical capacity of ca. 170 mAh/g, which is equally split between two one-electron voltage plateaus. Such high specific charges are rarely observed only in PBA samples with a low number of structural defects.
For example, the patented rhombohedral Na2MnFe(CN)6 displaying 150–160 mAh/g in capacity and a 3.4 V average discharge voltage[45][46][47] and rhombohedral Prussian white Na1.88(5)Fe[Fe(CN)6]·0.18(9)H2O displaying initial capacity of 158 mAh/g and retaining 90% capacity after 50 cycles.[48]
While Ti, Mn, Fe and Co PBAs show a two-electron electrochemistry, the Ni PBA shows only one-electron (Ni is not electrochemically active in the accessible voltage range). Iron-free PBA Na2MnII[MnII(CN)6] is also known. It has a fairly large reversible capacity of 209 mAh/g at C/5, but its voltage is unfortunately low (1.8 V versus Na+/Na).[49]
Electrolytes edit
Sodium-ion batteries can use aqueous and non-aqueous electrolytes. The limited electrochemical stability window of water results in lower voltages and limited energy densities. Non-aqueous carbonate ester polar aprotic solvents extend the voltage range. These include ethylene carbonate, dimethyl carbonate, diethyl carbonate, and propylene carbonate. The most widely used salts in non-aqueous electrolytes are NaClO4 and sodium hexafluorophosphate (NaPF6) dissolved in a mixture of these solvents. It is a well-established fact that these carbonate-based electrolytes are flammable, which pose safety concerns in large-scale applications. A type of glyme-based electrolyte, with sodium tetrafluoroborate as the salt is demonstrated to be non-flammable.[50] In addition, NaTFSI (TFSI = bis(trifluoromethane)sulfonimide) and NaFSI (FSI = bis(fluorosulfonyl)imide, NaDFOB (DFOB = difluoro(oxalato)borate) and NaBOB (bis(oxalato)borate) anions have emerged lately as new interesting salts. Of course, electrolyte additives can be used as well to improve the performance metrics.[51]
Comparison edit
Sodium-ion batteries have several advantages over competing battery technologies. Compared to lithium-ion batteries, sodium-ion batteries have somewhat lower cost, better safety characteristics, and similar power delivery characteristics, but also a lower energy density.
The table below compares how NIBs in general fare against the two established rechargeable battery technologies in the market currently: the lithium-ion battery and the rechargeable lead–acid battery.[36][52]
| Sodium-ion battery | Lithium-ion battery | Lead–acid battery | |
|---|---|---|---|
| Cost per kilowatt-hour of capacity | $40–77 (theoretical in 2019)[53] | $137 (average in 2020)[54] | $100–300[55] |
| Volumetric energy density | 250–375 W·h/L, based on prototypes[56] | 200–683 W·h/L[57] | 80–90 W·h/L[58] |
| Gravimetric energy density (specific energy) | 75–200 W·h/kg, based on prototypes and product announcements[56][59][60] | 120–260 W·h/kg (without protective case needed for battery pack in vehicle)[57] | 35–40 Wh/kg[58] |
| Power-to-weight ratio | ~1000 W/kg[61] | ~340-420 W/kg (NMC),[61] ~175-425 W/kg (LFP)[61] | 180 W/kg |
| Cycles at 80% depth of discharge[a] | Hundreds to thousands[1] | 3,500[55] | 900[55] |
| Safety | Low risk for aqueous batteries, high risk for Na in carbon batteries[63] | High risk[b] | Moderate risk |
| Materials | Earth-abundant | Scarce | Toxic |
| Cycling stability | High (negligible self-discharge) [citation needed] | High (negligible self-discharge) [citation needed] | Moderate (high self-discharge)[64] |
| Direct current round-trip efficiency | up to 92%[1] | 85–95%[65] | 70–90%[66] |
| Temperature range[c] | −20 °C to 60 °C[1] | Acceptable:−20 °C to 60 °C. Optimal: 15 °C to 35 °C[67] | −20 °C to 60 °C[68] |
Commercialization edit
Companies around the world have been working to develop commercially viable sodium-ion batteries. A 2-hour 5MW/10MWh grid battery was installed in China in 2023.[69]
Active edit
Altris AB edit
Altris AB was founded by Associate Professor Reza Younesi, his former PhD student, Ronnie Mogensen, and Associate Professor William Brant as a spin-off from Uppsala University, Sweden,[70] launched in 2017 as part of research efforts from the team on sodium-ion batteries. The research was conducted at the Ångström Advanced Battery Centre led by Prof. Kristina Edström at Uppsala University. The company offers a proprietary iron-based Prussian blue analogue for the positive electrode in non-aqueous sodium-ion batteries that use hard carbon as the anode.[71] Altris holds patents on non-flammable fluorine-free electrolytes consisting of NaBOB in alkyl-phosphate solvents, Prussian white cathode, and cell production. Clarios is partnering to produce batteries using Altris technology.[72]
BYD edit
The BYD Company is a Chinese electric vehicle manufacturer and battery manufacturer. In 2023, they invested $1.4B USD into the construction of a sodium-ion battery plant in Xuzhou with an annual output of 30 GWh.[73]
CATL edit
Chinese battery manufacturer CATL announced in 2021 that it would bring a sodium-ion based battery to market by 2023.[74] It uses Prussian blue analogue for the positive electrode and porous carbon for the negative electrode. They claimed a specific energy density of 160 Wh/kg in their first generation battery.[59] The company planned to produce a hybrid battery pack that includes both sodium-ion and lithium-ion cells.[75]
Faradion Limited edit
Faradion Limited is a subsidiary of India's Reliance Industries.[76] Its cell design uses oxide cathodes with hard carbon anode and a liquid electrolyte. Their pouch cells have energy densities comparable to commercial Li-ion batteries (160 Wh/kg at cell-level) with good rate performance till 3C and cycle lives of 300 (100% depth of discharge) to over 1,000 cycles (80% depth of discharge). Its battery packs have demonstrated use for e-bike and e-scooter applications.[36] They demonstrated transporting sodium-ion cells in the shorted state (at 0 V), eliminating risks from commercial transport of such cells.[77] It is partnering with AMTE Power plc[78] (formerly known as AGM Batteries Limited).[79][80][81][82]
In November 2019, Faradion co-authored a report with Bridge India[83] titled 'The Future of Clean Transportation: Sodium-ion Batteries'[84] looking at the growing role India can play in manufacturing sodium-ion batteries.
On December 5, 2022, Faradion installed its first sodium-ion battery for Nation in New South Wales Australia.[85]
HiNA Battery Technology Company edit
HiNa Battery Technology Co., Ltd is, a spin-off from the Chinese Academy of Sciences (CAS). It leverages research conducted by Prof. Hu Yong-sheng's group at the Institute of Physics at CAS. HiNa's batteries are based on Na-Fe-Mn-Cu based oxide cathodes and anthracite-based carbon anode. In 2023, HiNa partnered with JAC as the first company to put a sodium-ion battery in an electric car, the Sehol E10X. HiNa also revealed three sodium-ion products, the NaCR32140-ME12 cylindrical cell, the NaCP50160118-ME80 square cell and the NaCP73174207-ME240 square cell, with gravimetric energy densities of 140 Wh/kg, 145 Wh/kg and 155 Wh/kg respectively.[86] In 2019, it was reported that HiNa installed a 100 kWh sodium-ion battery power bank in East China.[87]
Chinese automaker Yiwei debuted the first sodium-ion battery-powered car in 2023. It uses JAC Group's UE module technology, which is similar to CATL's cell-to-pack design.[88] The car has a 23.2 kWh battery pack with a CLTC range of 230 kilometres (140 mi).[89]
KPIT Technologies edit
KPIT Technologies introduced India's first sodium-ion battery technology, marking a significant breakthrough in the country. This newly developed technology is predicted to reduce the cost of batteries for electric vehicles by 25-30%. It has been developed in cooperation with Pune's Indian Institute of Science Education and Research over the course of almost a decade and claims several notable benefits over existing alternatives such as lead-acid and lithium-ion. Among its standout features are a longer lifespan of 3,000–6,000 cycles, faster charging than traditional batteries, greater resistance to below-freezing temperatures and with varied energy densities between 100 and 170 Wh/Kg.[90][91][92]
Natron Energy edit
Natron Energy, a spin-off from Stanford University, uses Prussian blue analogues for both cathode and anode with an aqueous electrolyte.[93] Clarios is partnering to produce a battery using Natron technology.[94]
Northvolt edit
Northvolt, Europe's only large homegrown electric battery maker, has said it has made a "breakthrough" sodium-ion battery. Northvolt said its new battery, which has an energy density of more than 160 watt-hours per kilogram, has been designed for electricity storage plants but could in future be used in electric vehicles, such as two wheeled scooters.[5]
TIAMAT edit
TIAMAT spun off from the CNRS/CEA and a H2020 EU-project called NAIADES.[95] Its technology focuses on the development of 18650-format cylindrical cells based on polyanionic materials. It achieved energy density between 100 Wh/kg to 120 Wh/kg. The technology targets applications in the fast charge and discharge markets. Power density is between 2 and 5 kW/kg, allowing for a 5 min charging time. Lifetime is 5000+ cycles to 80% of capacity.[96][97][98][99]
They are responsible for one of the first commercialized product powered by Sodium-Ion battery technology, as of October 2023, through the commercialization of an electric screw-driver.[100]
SgNaPLus edit
SgNaPlus is a spin off from National University of Singapore, that uses a propeitary electrode and electrolyte. [1] It is based in Singapore and leverages on research conducted by Alternative Energy Systems Laboratory (AESL) from Energy and Bio-Thermal Systems Division in the Department of Mechanical Engineering, National University of Singapore (NUS)[2]. The division is founded by Prof Palani Balaya. SgNaPlus also has rights for the patent for a non-flammable sodium-ion batteries.
Defunct edit
Aquion Energy edit
Aquion Energy was (between 2008 and 2017) a spin-off from Carnegie Mellon University. Their batteries (salt water battery) were based on sodium titanium phosphate anode, manganese dioxide cathode, and aqueous sodium perchlorate electrolyte. After receiving government and private loans, the company filed for bankruptcy in 2017. Its assets were sold to a Chinese manufacturer Juline-Titans, who abandoned most of Aquion's patents.[101][102][100]
See also edit
- List of battery types
- Alkali metal-ion batteries:
- Lithium-ion battery
- Sodium-ion battery
- Potassium-ion battery
- Alkaline earth metal-ion batteries:
- Rechargeable battery
Notes edit
- ^ The number of charge-discharge cycles a battery supports depends on multiple considerations, including depth of discharge, rate of discharge, rate of charge, and temperature. The values shown here reflect generally favorable conditions.
- ^ See Lithium-ion battery safety.
- ^ Temperature affects charging behavior, capacity, and battery lifetime, and affects each of these differently, at different temperature ranges for each. The values given here are general ranges for battery operation.
References edit
External links edit
- Ma, Bingyuan; Lee, Youngju; Bai, Peng (2021). "Dynamic Interfacial Stability Confirmed by Microscopic Optical Operando Experiments Enables High-Retention-Rate Anode-Free Na Metal Full Cells". Advanced Science. 8 (12): 2005006. doi:10.1002/advs.202005006. ISSN 2198-3844. PMC 8224441. PMID 34194939.
- Wunderlich-Pfeiffer, Frank (April 19, 2023). "Na-ion: A battery worth its salt?". intercalationstation.substack.com. Retrieved 2023-04-28.
- Wu, Billy (January 3, 2024). Sodium ion batteries - The low-cost future of energy storage? (Podcast). Retrieved 2024-01-05.